Optimizing Split-Root Assays: A Strategic Guide to Enhancing Replicability and Robustness in Plant Science Research
This article provides a comprehensive framework for optimizing split-root assays, a crucial technique for studying systemic signaling and plant responses to heterogeneous environments.
Optimizing Split-Root Assays: A Strategic Guide to Enhancing Replicability and Robustness in Plant Science Research
Abstract
This article provides a comprehensive framework for optimizing split-root assays, a crucial technique for studying systemic signaling and plant responses to heterogeneous environments. We address the critical challenge of variability in multi-step protocols that can compromise replicability and robustness. Covering foundational principles, detailed methodologies, and troubleshooting strategies, this guide synthesizes current best practices for establishing robust assays in model organisms like Arabidopsis thaliana and extends to applications in crop species and hydroponic systems. By integrating recommendations for protocol documentation, validation techniques, and comparative analysis, this resource empowers researchers to generate reliable, repeatable, and biologically significant data, thereby accelerating discovery in plant nutrition, stress response, and drug development from natural compounds.
The Pillars of Reliability: Understanding Reproducibility, Replicability, and Robustness in Split-Root Assays
FAQs: Core Concepts and Definitions
What is the definitive difference between reproducibility and replicability in experimental biology?
In experimental biology, the terms are distinct, though often confused. Under the widely used Claerbout/Donoho/Peng convention:
- Reproducibility is the ability to recreate identical results using the original data and analysis methods, a standard now considered a minimal requirement for research [1] [2].
- Replicability is the ability to obtain consistent results when a new study is performed, using new data but the same experimental methods and conditions. This is more challenging in biological systems due to inherent biological variability and experimental noise [1] [3].
How is 'robustness' different from replicability?
Robustness is the capacity of an experimental outcome to remain consistent despite slight variations or deviations in the experimental protocol. While replicability tests whether you can get the same result under the same conditions, robustness tests whether the finding holds true under different but related conditions. A robust finding is more likely to be biologically significant and relevant under natural, variable environments [1].
Why is robustness particularly important for complex protocols like split-root assays?
Complex, multi-step protocols like split-root assays naturally have numerous points of variation (e.g., growth media, light levels, recovery time). Investigating robustness helps identify which protocol details are critical and which allow for flexibility. This is crucial because:
- It enhances the potential for other labs with different equipment or resources to perform similar research [1].
- Robust outcomes are more likely to represent fundamental biological phenomena rather than artifacts of a specific protocol [1].
Troubleshooting Guide: Enhancing Replicability and Robustness in Split-Root Assays
Split-root assays are powerful for studying systemic and local signaling in plants but present significant challenges in achieving replicable and robust results. Below are common issues and evidence-based solutions.
Problem: Low Survival Rates or Stunted Growth After Root Splitting
This is a common issue that threatens the validity of the entire experiment.
- Potential Cause: Excessive root system trauma during the splitting procedure. The method of "total de-rooting" (cutting at the shoot-to-root junction) is highly stressful [4].
- Recommended Solution: Adopt a "partial de-rooting" method.
- Protocol Detail: Instead of cutting at the shoot-to-root junction, make the cut approximately half a centimeter below, leaving a part of the main root attached [4].
- Evidence: A detailed methodological study compared total de-rooting (TDR) and partial de-rooting (PDR) in Arabidopsis thaliana. The results strongly favor PDR [4]:
| De-rooting Method | Recovery Time | Final Rosette Area | Survival Rate |
|---|---|---|---|
| Partial De-rooting (PDR) | Significantly shorter | Much closer to uncut plants | Much higher |
| Total De-rooting (TDR) | Significantly longer | Extremely decreased | Lower, especially at 9-11 DAS |
Problem: Inconsistent Phenotypic Outcomes Across Replicates
Different labs observe different results even when trying to follow the same published method.
- Potential Cause: Uncontrolled variation in key experimental parameters. Published methods often omit which details are critical versus flexible [1].
- Recommended Solution: Systematically document and, if possible, test the robustness of your protocol to variations.
- Actionable Steps:
- Extend Documentation: Record more detail than you think is necessary in your lab journal, including minor deviations [1].
- Communicate with Original Authors: When replicating a study, contact the original authors for clarification. Templates are available to facilitate this communication professionally [5].
- Consult Comparative Tables: Use published resources that compile protocol variations. For example, the table below shows the diversity in split-root protocols for nitrate foraging in Arabidopsis, all of which successfully demonstrated preferential foraging, a robust outcome [1]:
- Actionable Steps:
| Publication | HN Concentration | LN Concentration | Sucrose in Media | Days Before Cutting | Recovery Period |
|---|---|---|---|---|---|
| Ruffel et al. (2011) | 5 mM KNO₃ | 5 mM KCl | 0.3 mM | 8-10 days | 8 days |
| Remans et al. (2006) | 10 mM KNO₃ | 0.05 mM KNO₃ | None | 9 days | None |
| Poitout et al. (2018) | 1 mM KNO₃ | 1 mM KCl | 0.3 mM | 10 days | 8 days |
| Girin et al. (2010) | 10 mM NH₄NO₃ | 0.3 mM KNO₃ | 1% | 13 days | None |
Problem: Failure to Replicate a Specific Systemic Signaling Phenotype
You observe the basic preferential foraging effect, but fail to replicate more nuanced reported findings, such as specific growth comparisons to homogeneous controls.
- Potential Cause: The specific phenotype may be highly sensitive to particular protocol parameters and lack robustness. The seminal finding by Ruffel et al. (2011) that the HN side in a heterogeneous setup grows more than the HN side in a homogeneous setup (HNln > HNHN) may be less robust than the basic foraging response [1].
- Recommended Solution:
- Troubleshoot Key Variables: Focus on factors known to influence systemic signaling, such as the nitrogen source in the growth media prior to splitting and the duration of the recovery period after splitting [1].
- Contextualize Findings: A failure to replicate a less robust finding does not necessarily invalidate your experiment. It can help the community map the boundary conditions of a biological phenomenon [1] [2].
The Scientist's Toolkit: Research Reagent Solutions for Split-Root Assays
The following table details key materials used in establishing split-root systems across different plant species.
| Item | Function | Application Note |
|---|---|---|
| Clone Collars | Supports the plant shoot while allowing roots to grow into a hydroponic solution. | Must be sterilized (e.g., with ethanol) before use to prevent contamination [6]. |
| Hydroponic Beakers | Vessel for growing split-root systems under controlled nutrient conditions. | 250 ml glass beakers are commonly used for Arabidopsis and pine seedlings [4] [6]. |
| Solid Growth Medium (Agar) | A stable substrate for initial seedling growth and root development prior to splitting. | Often contains a low concentration of sucrose (e.g., 0.3-1%) as a carbon source [1]. |
| SafeT-Sorb | An inorganic, solid substrate used as a potting medium for later growth stages. | Provides physical support and is inert, minimizing unintended nutritional interactions [6]. |
| Nitrogen Sources (KNO₃, KCl) | Used to create high-nitrogen (HN) and low-nitrogen (LN) environments for different root halves. | KCl is often used as an osmotic control in the LN compartment [1]. |
| Chemical Reagent | ||
| 5-Lox-IN-3 | 5-Lox-IN-3, MF:C19H16ClN5O, MW:365.8 g/mol | Chemical Reagent |
Experimental Workflow and Signaling Pathways
The following diagrams outline the logical relationships between the critical triad concepts and a generalized workflow for a split-root experiment.
Conceptual Relationship of the Critical Triad
Split-Root Assay Workflow for Systemic Signaling
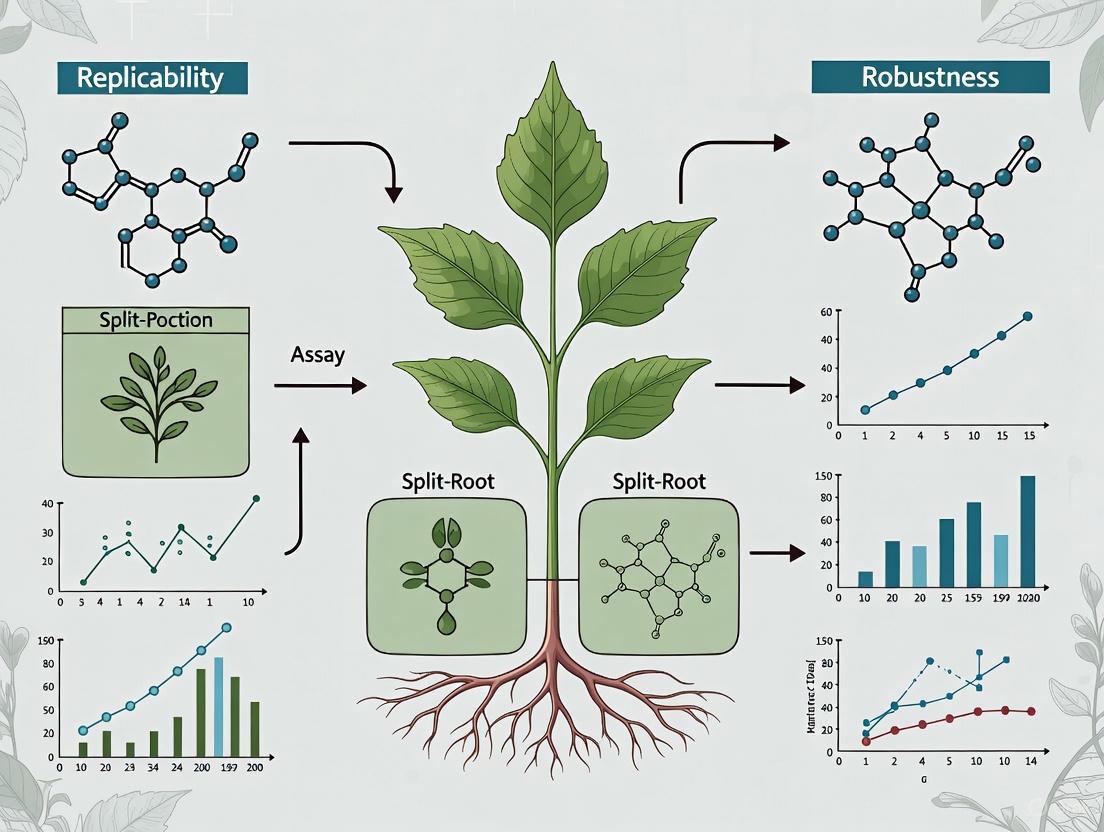
The split-root assay is a sophisticated plant research technique where a root system is physically divided and placed into two or more separate, isolated compartments. This setup allows researchers to apply different treatments to each part of the root system while the plant shares a common shoot system [4]. The primary power of this method is its ability to discriminate between local responses (occurring at the site of the treatment) and systemic responses (signals that travel to and affect other parts of the plant) [1]. This is vital for unraveling complex long-distance signaling pathways in plants, which play a central role in processes like nutrient foraging, drought adaptation, and interactions with soil organisms [1] [7] [8].
The technique's versatility allows for its application in a wide range of studies, from investigating the systemic signaling pathways that indicate the demand for and local supply of nutrients [1], to analyzing plant responses to drought [4], ion transport regulation [7], and colonization by mycorrhizal fungi [7].
Establishing a Split-Root System: Methodologies and Protocols
Several methods exist for establishing a split-root system (SRS), each with advantages and disadvantages depending on the plant species and research goals. The choice of method is critical for the success and interpretability of the experiment.
Common Methods for Generating Split-Root Systems
The table below summarizes the primary methods used to create split-root systems, particularly focusing on small model plants like Arabidopsis thaliana and the specific considerations for woody plants [4] [7].
Table 1: Methods for Establishing Split-Root Systems
| Method Name | Description | Best For | Key Advantages | Key Disadvantages/Limitations |
|---|---|---|---|---|
| Split Newly Forming Roots (SNR) [4] [7] | The main root is cut to induce the formation of lateral roots, which are then separated into different compartments. | Young seedlings of species with a single primary root (e.g., Arabidopsis). | Allows establishment in young plants [4]. | Physically damages the plant, which can induce stress responses and make plants more susceptible to pathogens [7]. |
| Partial vs. Total De-rooting [4] | A variant of SNR where the cut is made either partway down the main root (Partial De-rooting, PDR) or at the shoot-to-root junction (Total De-rooting, TDR). | Early establishment of SRS in young plants. | PDR leads to a shorter recovery time, higher survival rate, and less stress than TDR [4]. | TDR imposes greater stress, leading to extended recovery and reduced final plant size [4]. |
| Split-Developed Root (SDR) [7] | A well-developed root system is divided into two parts of comparable size and placed in separate containers. | Older plants, woody species without a dominant taproot. | Simple and easy to perform; useful for testing horizontal soil heterogeneity [7]. | Difficult to apply to plants with a strong taproot; can cause significant root damage [7]. |
| Grafting Techniques [7] | Using horticultural techniques like "inverted Y-grafting" to attach a second root system from another plant. | Species that graft well; studies requiring genetically distinct rootstocks. | Allows experimentation with plants forming a taproot; can combine different genotypes [7]. | Very skill-demanding; grafted plants often have low survivability rates [4] [7]. |
Key Experimental Variations in Split-Root Protocols
Even for a specific application like nitrate foraging in Arabidopsis, published protocols show extensive variation. The table below, derived from a 2025 review, highlights the diversity in key parameters across studies, all of which successfully demonstrated the core preferential foraging response [1].
Table 2: Protocol Variations in Arabidopsis Split-Root Nitrate Foraging Assays
| Parameter | Example Variations from Literature |
|---|---|
| Nitrogen Concentration | High Nitrate (HN): 1 mM to 10 mM KNO₃ or NH₄NO₃; Low Nitrate (LN): 0.05 mM KNO₃ to 10 mM KCl [1] |
| Growth Media | Sucrose: 0% to 1%; Nitrogen Source: Varies (e.g., NHâ‚„âº-succinate, KNO₃) [1] |
| Environmental Conditions | Light Intensity: 40 to 260 mmol mâ»Â² sâ»Â¹; Photoperiod: Long day or Short day; Temperature: 21°C to 22°C [1] |
| Protocol Timing | Days before cutting: 6-13 days; Recovery period: 0-8 days; Heterogeneous treatment: 5-7 days [1] |
Troubleshooting Common Experimental Challenges
FAQ 1: Our split-root plants show stunted growth and low survival rates after the procedure. What can we do?
- Problem: The de-rooting process is causing excessive stress.
- Solution: Implement the Partial De-rooting (PDR) method instead of Total De-rooting (TDR). Research shows that cutting the main root approximately half a centimeter below the shoot-to-root junction, rather than right at the junction, significantly shortens the recovery time, improves the final rosette area, and dramatically increases survival rates [4]. Ensure the procedure is performed on plants at the optimal developmental stage, as survival can drop if performed when all leaves are of very similar size [4].
FAQ 2: We cannot replicate a published systemic signaling phenotype in our hands. Where should we look for the issue?
- Problem: The experimental outcome is not robust to slight variations in the protocol.
- Solution: Systematically compare and adjust your protocol parameters against the literature. As shown in Table 2, factors like light intensity, sucrose concentration in the media, and the duration of recovery and treatment periods vary widely and can significantly impact results [1]. Document and report all these parameters in detail. Furthermore, strive to investigate and report on the robustness of your own protocols—identifying which parameters are critical and which can be flexible will greatly aid replicability [1].
FAQ 3: How can we be sure that our split-root compartments are truly hydraulically isolated?
- Problem: Water or solutes may be moving between compartments, confounding the interpretation of local vs. systemic effects.
- Solution: Use physical barriers like wax or paraffin layers that are penetrable by roots but designed to prevent bulk water flow [8]. However, be aware that these layers can increase resistance to vertical water flow and affect plant water status and growth [8]. It is critical to validate the isolation, for example, by using tracer dyes or through mechanistic modeling that can account for and simulate potential leakage [8].
FAQ 4: What is the impact of the split-root procedure itself on plant physiology?
- Problem: The surgical stress of the procedure may alter the plant's baseline state, affecting its response to subsequent treatments.
- Solution: Always include appropriate controls, such as sham-operated plants or uncut plants. Be aware that proteomic analyses have revealed that the de-rooting procedure triggers distinct metabolic alterations in leaves during the healing process [4]. The plant's adaptation to the SRS may change its tolerance to stresses like drought or pathogen attack, which must be considered when interpreting results [4].
The Scientist's Toolkit: Essential Research Reagents and Materials
Table 3: Key Research Reagents and Materials for Split-Root Assays
| Item | Function/Application in Split-Root Assays |
|---|---|
| Agar Plates | A common growth medium for SRS in small plants like Arabidopsis; allows precise control of nutrient content and easy visualization of roots [1]. |
| Physical Dividers/Partitions | Used to create separate root environments within a single pot or agar plate, ensuring physical and hydraulic isolation of root compartments [4]. |
| Nitrate Salts (KNO₃, NH₄NO₃) | Used to create high and low nitrate treatments for studying systemic nutrient signaling and preferential root foraging [1]. |
| Inert Salts (KCl, Kâ‚‚SOâ‚„) | Often used as osmotic controls in the low-nitrate compartment to substitute for nitrate salts and isolate the nitrogen-specific response [1]. |
| Sucrose | A carbon source added to the growth media in some protocols; its concentration (e.g., 0.3% to 1%) is a notable variable that can affect plant growth and experimental outcomes [1]. |
| Paraffin/Wax Layers | Used as hydraulically isolating barriers in soil-based systems to create strong moisture gradients between compartments and prevent water redistribution [8]. |
| Tuberculosis inhibitor 11 | Tuberculosis inhibitor 11, MF:C29H37N3O9, MW:571.6 g/mol |
| Antibacterial agent 170 | Antibacterial agent 170, MF:C14H9Cl2NO2S, MW:326.2 g/mol |
Visualizing Systemic Signaling in Split-Root Assays
The following diagram illustrates the core logic and experimental workflow of a split-root assay designed to investigate systemic signaling, using nutrient foraging as an example.
The split-root assay remains an indispensable tool for deciphering the complex language of systemic signaling in plants. Its successful application, however, hinges on a deep understanding of its methodological nuances. Challenges in achieving robust, replicable results often stem from the extensive variation in protocols and the inherent stress of the procedure itself [1] [4]. By adopting less stressful methods like partial de-rooting, meticulously documenting all protocol parameters, systematically investigating which variations critically impact outcomes, and using appropriate controls, researchers can significantly enhance the reliability and robustness of their findings [1] [4]. This rigorous approach ensures that the split-root assay continues to yield fundamental insights into plant biology that are both reproducible and meaningful.
For scientific progress, especially in fields with complex biological experiments and drug development, the reliability of research outcomes is paramount. This reliability stands on three pillars: reproducibility, replicability, and robustness.
- Reproducibility is the ability to recreate quantitatively identical results using the same raw data, methods, and computational codes [1].
- Replicability refers to obtaining statistically similar results when an experiment is repeated under the same conditions, acknowledging inherent biological and experimental noise [1].
- Robustness, in the context of experimental biology, is the capacity to generate similar scientific outcomes despite slight variations in experimental protocols or conditions [1].
A robust experimental outcome is more likely to represent a significant biological phenomenon relevant in natural, variable environments, rather than being an artifact of a specific, finely-tuned laboratory setup. Furthermore, robustness enhances the broader applicability of research, making it more accessible to labs with different equipment or funding levels [1].
Split-Root Assays: A Case Study in Robustness
Split-root assays, where a plant's root system is divided and exposed to different environments, are a powerful tool for studying local and systemic signaling in plant responses to nutrients and abiotic stresses [1] [4]. The complexity of these multi-step experiments, however, makes them a prime case study for investigating robustness.
Key Methodologies for Establishing Split-Root Systems
Several methods exist for creating split-root systems, each with advantages and limitations. The choice of method can significantly impact the robustness of subsequent results.
- Partial vs. Total De-rooting: A key methodological consideration is the point of the initial root cut. Research shows that a partial de-rooting (cutting the main root about half a centimeter below the shoot-to-root junction) is superior to total de-rooting (cutting at the junction). Partial de-rooting leads to a shorter recovery time, a final rosette area closer to uncut plants, and a higher survival rate, indicating it is a less stressful procedure for the plant [4].
- Grafting Technique: For some species like cotton, a grafting method can be used. This involves making an incision in the hypocotyl of one seedling and inserting the rootstock from another, resulting in a plant with two root systems. This method can achieve survival rates over 95% [9].
- Hydroponic Protocol: Recent work in upland cotton has established a rapid hydroponic protocol where the primary root is cut and the seedling is transplanted to encourage lateral root growth. This system was validated across eight varieties, showing no significant difference in root dry weight between the two halves, confirming its reliability [10].
Documented Variations in Split-Root Protocols
A survey of published literature on Arabidopsis thaliana split-root assays for nitrate foraging reveals extensive variation in nearly every aspect of the protocol. The table below summarizes these differences, all of which nonetheless reported the core observation of preferential foraging [1].
Table: Documented Variations in Arabidopsis Split-Root Assay Protocols
| Parameter | Example Variations from Literature |
|---|---|
| High Nitrate (HN) Concentration | 1 mM KNO₃ to 10 mM KNO₃ [1] |
| Low Nitrate (LN) Concentration | 0.05 mM KNO₃ to 10 mM KCl [1] |
| Days Before Cutting | 6 days to 13 days after sowing [1] |
| Recovery Period | No recovery period to 8 days [1] |
| Heterogeneous Treatment Duration | 5 days to 7 days [1] |
| Sucrose in Media | None to 1% [1] |
| Light Intensity | 40 µmol mâ»Â² sâ»Â¹ to 260 µmol mâ»Â² sâ»Â¹ [1] |
FAQs and Troubleshooting for Robust Split-Root Experiments
Frequently Asked Questions
Q1: What is the most critical step for ensuring a healthy split-root plant in Arabidopsis? A: The type of initial cut is crucial. We strongly recommend partial de-rooting over total de-rooting. This method leaves a portion of the main root attached, minimizing stress, reducing recovery time, and significantly increasing survival rates and subsequent growth [4].
Q2: Our lab cannot replicate a published split-root protocol exactly due to equipment constraints. Does this mean the experiment is doomed to fail? A: Not necessarily. This is where robustness is key. While exact replication is ideal, a robust biological phenomenon should withstand moderate variations in parameters like light intensity or media composition. The variations summarized in Table 1 show that the preferential foraging phenotype is observed across a wide range of conditions. Focus on replicating the core experimental logic rather than every minor parameter [1].
Q3: Why is it important to report even the seemingly minor details of my protocol, such as the exact brand of agar or time of day the transfer was performed? A: The "unknown robustness" of a protocol is a major hurdle for replicability. A detail that seems minor in your lab might be critical for success in another context. Comprehensive reporting allows others to identify which parameters are flexible and which are essential, building a collective knowledge base for robust methodology [1].
Q4: How can I use a split-root system to study drought stress without causing rehydration during compound application? A: A split-root system can be adapted for this purpose. Grow the plant with both halves of the root system in water-deprived conditions. When applying a water-soluble compound, add it only to one half of the root system. Once the compound is absorbed, you can sever that specific root section to minimize rehydration of the entire plant, thereby maintaining the drought stress conditions [4].
Troubleshooting Common Problems
Table: Troubleshooting Guide for Split-Root Assays
| Problem | Potential Causes | Solutions |
|---|---|---|
| Low survival rate after cutting | - Total de-rooting causing excessive stress.- Plants at a suboptimal developmental stage (e.g., 4-leaf stage for Arabidopsis).- Hypocotyl not in contact with growth medium. | - Switch to a partial de-rooting method [4].- Perform the procedure at the 2-true-leaf stage [9].- Ensure the cut end remains in contact with the medium. |
| High variability in root growth between halves | - Unequal splitting of the root system.- Physical damage to one side during setup.- Inconsistent environmental conditions (e.g., light, temperature) between compartments. | - Use a grafting method to ensure two uniform root systems [9].- Handle roots gently with sterilized tools.- Ensure both compartments are in a randomized, uniform growth environment. |
| Failure to observe expected systemic phenotype | - Insufficient recovery time after splitting.- Inconsistent application of treatments.- The phenotype may not be robust to your specific protocol variations. | - Extend the recovery period until plants resume normal growth rates [4].- Double-check treatment concentrations and compartment isolation.- Consult literature for robust phenotypes (e.g., HNln > LNhn) and compare your protocol variations to published ones [1]. |
The Researcher's Toolkit: Essential Materials for Split-Root Assays
Table: Key Research Reagent Solutions for Split-Root Experiments
| Item | Function/Application | Example Details |
|---|---|---|
| Agar/Growth Media | Solid support and nutrient delivery for in vitro systems. | Composition varies (e.g., with/without sucrose; different N sources like NH4+-succinate or KNO3) [1]. |
| Hydroponic Nutrient Solution | For liquid-based culture systems. | Typically contains macro- and micronutrients (e.g., Ca(NO₃)₂, KNO₃, MgSO₄, MgSO₄, H₃BO₃); pH adjusted to ~6.0 [9]. |
| Sterilized Sand | A substrate for germination and early growth, especially for species like cotton. | Used in plastic boxes for initial seedling growth before grafting or transfer [9]. |
| Parafilm | Sealing and grafting aid. | Used to closely wrap and secure grafted seedlings to prevent wilting and maintain humidity [9]. |
| Aeration Instrument | Oxygenation of hydroponic solutions. | Critical for maintaining oxygen levels in nutrient solutions for root health, especially in deep vessels [9]. |
| DNA-PK-IN-10 | DNA-PK-IN-10, MF:C25H28N6O2, MW:444.5 g/mol | Chemical Reagent |
| Taurodeoxycholic acid-d4 | Taurodeoxycholic acid-d4 Sodium Salt|Internal Standard | Taurodeoxycholic acid-d4 is a high-purity, deuterated internal standard for precise bioanalytical quantification. This product is for Research Use Only (RUO). Not for human or veterinary use. |
Workflow and Conceptual Framework
The following diagrams illustrate the core experimental workflow for a robust split-root assay and the conceptual relationship between robustness, replicability, and broader relevance.
Split-Root Experimental Workflow
Robustness Drives Relevance and Applicability
Troubleshooting Guides
FAQ 1: Why is there low survival and high variability in my split-root seedlings post-surgery?
The survival rate and subsequent growth of seedlings are highly dependent on the de-rooting technique and the developmental stage at which the procedure is performed.
- Problem: Low survival rate and extended recovery time for seedlings after establishing the split-root system.
- Root Cause: The method of de-rooting (total vs. partial) and the timing of the procedure are critical. Total de-rooting, which involves cutting the root at the shoot-to-root junction, imposes significant stress on the plant. Performing this procedure at certain developmental stages (e.g., the four-leaf stage) can make it difficult to keep the hypocotyl in contact with the growth medium, leading to plant death [4].
- Solution:
- Implement Partial De-Rooting: Instead of a total cut at the shoot-to-root junction, cut the main root approximately half a centimeter below the junction. This leaves a part of the main root attached and significantly reduces stress [4].
- Optimize Timing: Perform the procedure at a developmental stage that facilitates recovery. Evidence suggests that partial de-rooting leads to a much shorter recovery time and a final rosette area closer to that of uncut plants compared to total de-rooting [4].
FAQ 2: Why do I get inconsistent root foraging responses despite using established protocols?
Inconsistent phenotypic outcomes, such as preferential root foraging, can often be traced back to unintentional variations in the components and conditions of the growth media.
- Problem: Inconsistent or unreproducible root foraging responses (e.g., preferential investment in root growth in high-nitrate compartments) when following a published split-root protocol.
- Root Cause: Published protocols for similar experiments, such as nitrate foraging in Arabidopsis thaliana, can vary extensively in key media components and environmental conditions. These variations include the concentration of nitrogen sources, sucrose levels, light intensity, photoperiod, and the duration of growth steps [1]. Without knowing which of these parameters are flexible and which are critical, replicating results is challenging.
- Solution:
- Systematic Parameter Checking: Carefully compare all aspects of your protocol against the original method. Pay close attention to the concentrations of all media components, not just the primary treatment [1].
- Extend Protocol Detail: When publishing, include exhaustive detail on every aspect of the protocol, noting which parameters were optimized and which can be varied without affecting the core outcome. This enhances the robustness and replicability of the research for others [1].
FAQ 3: How can I improve the consistency of halotropism assays using a split-agar system?
Inconsistency in the initiation point of the treatment can be a major source of variability in tropism assays.
- Problem: High variability in root bending responses in halotropism (salt avoidance) assays using a split-agar system.
- Root Cause: In traditional methods, seeds are germinated directly on a plate where a salt gradient is later established. This leads to inconsistency in root lengths at the time of treatment, meaning the distance between the root tip and the treatment interface varies greatly [11].
- Solution:
- Standardize Seedling Transfer: Do not germinate seeds directly on the treatment plate. Instead, germinate and grow seedlings on a standard medium (e.g., 1/2 MS) for a set period (e.g., 4–5 days). Then, selectively transfer seedlings of comparable root length to the pre-prepared split-agar assay plate. This ensures a uniform starting point and distance to the salt gradient for all seedlings, improving the efficiency and consistency of phenotyping [11].
FAQ 4: My split-root system is not establishing two equal root halves. What should I do?
Uneven root systems can compromise the experimental design where two distinct environments are being compared.
- Problem: Failure to develop a split-root system with two relatively equal root halves, leading to an imbalance in the experimental setup.
- Root Cause: Insufficient promotion of lateral root growth before splitting, or an unsuitable technique for the plant species.
- Solution:
- For Arabidopsis and similar species: Use the partial de-rooting method to encourage more uniform lateral root development from the remaining root stub [4].
- For species like Loblolly Pine: Implement a hydroponic system. One month after germination, sever the primary root tip and grow the seedlings in a hydroponic medium for several weeks. This technique actively promotes the elongation of lateral roots to a length suitable for easy division into two equal parts [6].
Summarized Quantitative Data from Literature
The table below compiles key variations in split-root protocols from published studies on nitrate foraging, highlighting potential sources of variability.
Table 1: Protocol Variations in Arabidopsis Split-Root Nitrate Foraging Assays [1]
| Paper | HN Concentration | LN Concentration | Photoperiod & Light Intensity | Days Before Cutting | Recovery Period | Sucrose Concentration |
|---|---|---|---|---|---|---|
| Ruffel et al. (2011) | 5 mM KNO₃ | 5 mM KCl | Long day - 50 μmol mâ»Â² sâ»Â¹ | 8-10 days | 8 days | 0.3% |
| Remans et al. (2006) | 10 mM KNO₃ | 0.05 mM KNO₃ | Long day - 230 μmol mâ»Â² sâ»Â¹ | 9 days | None | None |
| Poitout et al. (2018) | 1 mM KNO₃ | 1 mM KCl | Short day - 260 μmol mâ»Â² sâ»Â¹ | 10 days | 8 days | 0.3% |
| Tabata et al. (2014) | 10 mM KNO₃ | 10 mM KCl | Long day - 40 μmol mâ»Â² sâ»Â¹ | 7 days | 4 days | 0.5% |
Experimental Workflow and Methodology
Graphical Abstract: Split-Root Establishment via Partial De-Rooting
Key Steps:
- Plant Material: Surface-sterilize Arabidopsis thaliana seeds and sow on vertical plates containing standard growth medium (e.g., 1/2 MS). Stratify at 4°C for 2-3 days, then transfer to a controlled growth chamber [11] [6].
- Initial Growth: Grow seedlings vertically until the primary root has developed two lateral roots of sufficient potential for splitting.
- Partial De-Rooting: Using a sterile scalpel or razor blade, make a clean cut on the primary root approximately 0.5 cm below the shoot-to-root junction. This leaves a portion of the primary root attached, which minimizes stress compared to a total cut at the junction [4].
- Recovery Phase: Transfer the cut seedling to a fresh recovery medium. Allow the plant to recover and the lateral roots to elongate. Plants treated with partial de-rooting demonstrate a significantly shorter recovery time and higher survival rates than totally de-rooted plants [4].
- Splitting: Once two lateral roots are long enough, carefully guide them into two separate physical compartments (e.g., two pots, or two sides of a split-plate).
- Differential Treatment: After the split-root system is fully established, apply the experimental treatments to the respective compartments.
The Scientist's Toolkit: Essential Research Reagents
Table 2: Key Reagents for Split-Root Assays
| Item | Function in the Protocol | Example & Context |
|---|---|---|
| Basal Salt Mixture | Provides essential macro and micronutrients for plant growth. The foundation of the growth medium. | Murashige & Skoog (MS) Basal Salt Mixture is widely used for Arabidopsis and other plants in vitro [11] [6]. |
| Agar | A gelling agent that provides solid support for root growth on plates. | High-purity agar at a concentration of ~1.2% is typical for creating solid growth media [11]. |
| Nitrogen Sources | Key treatment variable for nutrient foraging studies. Forms include nitrate, ammonium, or their salts. | KNO₃ and KCl are often used as High Nitrogen (HN) and Low Nitrogen (LN) sources, respectively [1]. |
| Sucrose | A carbon source added to the medium to support plant growth, especially in vitro. | Concentrations vary between protocols (e.g., 0.3%, 0.5%, 1%, or none), impacting plant metabolism and potentially the experimental outcome [1]. |
| Sodium Hypochlorite | Used for surface sterilization of seeds to prevent microbial contamination. | A 5% (v/v) solution is commonly used for a 10-minute sterilization step [11] [6]. |
| Plant Growth Regulators | Used to study specific signaling pathways or to modify root architecture. | Auxins like IAA (Indole-3-acetic acid) or inhibitors like NPA (N-1-Naphthylphthalamic acid) can be used in tropism studies [11]. |
| Cbl-b-IN-15 | Cbl-b-IN-15, MF:C27H27N5O3, MW:469.5 g/mol | Chemical Reagent |
| DosatiLink-1 | DosatiLink-1|ABL Enzyme Inhibitor | DosatiLink-1 is a potent Abelson murine leukemia (ABL) enzyme inhibitor for research. This product is for Research Use Only (RUO). Not for human use. |
Systemic Signaling Pathway in Split-Root Experiments
The diagram below illustrates the conceptual signaling pathways investigated using split-root systems, where local and systemic signals are integrated to regulate root growth.
Conceptual Model: Local and Systemic Signaling in a Split-Root Setup
From Theory to Practice: Establishing Robust Split-Root Systems Across Species and Platforms
For researchers investigating systemic signaling, nutrient foraging, and plant responses to heterogeneous environments, split-root assays are an indispensable tool. These techniques allow for the physical separation of a single plant's root system into distinct compartments, enabling the study of local versus systemic signaling in a controlled manner. The reliability and robustness of this research hinge on selecting and correctly implementing the appropriate setup. This guide provides a detailed comparison of de-rooting, grafting, and hydroponic methodologies to help you optimize your split-root experiments for maximum replicability and robust outcomes.
FAQs: Core Concepts and Setup Selection
What is the primary scientific value of using a split-root system?
Split-root systems are primarily used to differentiate between local and systemic plant responses. When a plant's root system is divided and exposed to different conditions in each compartment, researchers can determine whether a plant's reaction—be it to nutrients, drought, salinity, or pathogens—is confined to the roots in direct contact with the stimulus (local) or if a signal is sent through the plant, triggering a whole-organism response (systemic). This is fundamental for understanding plant signaling, nutrient foraging, and stress adaptation mechanisms [1] [4].
Which setup method is least disruptive for small model plants like Arabidopsis?
For small model plants such as Arabidopsis thaliana, the Partial De-Rooting (PDR) method is significantly less disruptive than total de-rooting. Evidence shows that PDR results in a shorter recovery time, a final rosette area much closer to that of uncut plants, and a higher survival rate compared to Total De-Rooting (TDR). The minimal leftover root tissue in PDR helps maintain water and nutrient uptake during recovery, reducing overall stress and leading to more reliable experimental data [4].
When should I consider the grafting method?
The grafting method is particularly valuable for creating a split-root system in species with a single primary root where other methods are challenging, or for studies requiring a very uniform distribution of root biomass between compartments from the outset. For example, a protocol for cotton involves grafting two seedlings together at the hypocotyl, creating a plant with two genetically identical and uniform root systems. This method boasts a high reported survival rate of over 95% [9]. However, it is a skill-demanding technique.
How do hydroponic systems enhance split-root research?
Hydroponic systems offer superior control over the root environment, which is crucial for replicability. Unlike soil or sand, hydroponics allows for precise regulation of nutrient concentrations, pH, and other abiotic variables. This minimizes environmental "noise," leading to more consistent and interpretable results. Furthermore, these systems facilitate easy and clean harvesting of root tissues for downstream molecular analyses like transcriptomics and metabolomics [12] [13]. Scalable systems can be created using common lab materials like PCR strip tubes and pipette tip boxes [13].
Troubleshooting Guides
Issue 1: Low Survival Rate After De-Rooting
Problem: A high percentage of seedlings die after the de-rooting procedure.
Solutions:
- Switch to Partial De-Rooting: If you are using total de-rooting (cutting at the shoot-root junction), modify your technique to make the cut approximately 0.5 cm below the junction, leaving a small portion of the main root attached. This dramatically improves survival and recovery [4].
- Optimize Developmental Stage: Perform the de-rooting procedure at the correct developmental stage. For Arabidopsis, cutting at 11 or 15 days after sowing (DAS) can sharply reduce final plant health in TDR plants. Aim for an earlier stage, as guided by established protocols [4].
- Ensure Hypocotyl Contact: After de-rooting, ensure the cut end of the hypocotyl remains in good contact with the growth medium to uptake water and nutrients [4].
Issue 2: Lack of Robustness and Replicability in Results
Problem: Experimental outcomes are not consistent across replicates or when slightly varying the protocol.
Solutions:
- Formalize Protocol Details: Document and adhere to every detail of your protocol, as slight variations in factors like light intensity, sucrose concentration in media, and duration of growth steps can significantly impact outcomes [1].
- Validate Root Uniformity: When using methods that involve splitting lateral roots, validate that the two root halves are statistically similar in biomass before applying treatments. For example, a protocol for cotton confirmed no significant difference in root dry weight between the two halves across eight varieties, ensuring that any observed effects are due to the treatment and not initial asymmetry [12].
- Control for Procedure Stress: Acknowledge that the de-rooting or grafting procedure itself is a stressor. A proteomic analysis revealed that totally and partially de-rooted plants undergo distinct metabolic alterations during healing. Using the less stressful PDR method minimizes this confounding factor [4].
Issue 3: Challenges in Scaling or Maintaining Sterility
Problem: The system is difficult to scale for high-throughput studies, or microbial contamination is common.
Solutions:
- Adopt a Scalable Hydroponic Design: Implement a compact hydroponic system built from 8-strip PCR tubes and a 96-well pipette tip box reservoir. This design is modular, cost-effective, and supports sterile or semi-sterile long-term cultivation, making it ideal for high-throughput phenotyping [13].
- Follow Aseptic Protocols: For laboratory-oriented studies, strictly follow aseptic techniques. This includes autoclaving tools, nutrient solutions, and substrates, sterilizing seeds, and using biosafety cabinets for procedures to minimize microbial contamination [12].
Comparative Data at a Glance
The table below summarizes the key characteristics of the primary split-root setup methods to aid in your selection.
| Method | Best For | Key Advantage | Key Disadvantage | Evidence of Robustness |
|---|---|---|---|---|
| Partial De-Rooting (PDR) | Small plants (e.g., Arabidopsis); early establishment | Minimal stress; short recovery time; high survival rate | Requires precision cutting | Final rosette area close to uncut plants; distinct, less severe proteomic response vs. TDR [4] |
| Total De-Rooting (TDR) | Species where PDR is not feasible | Complete removal of primary root | High stress; long recovery; lower survival | Extended recovery time; significant reduction in final rosette area, especially if performed later [4] |
| Grafting | Species with a single primary root (e.g., cotton) | Creates two highly uniform root systems from two seedlings | Skill-demanding; potential for low survivability | Survival rate >95%; successful for studying salt stress distribution [9] |
| Hydroponic Split-Root | Nutrient signaling, molecular analysis; high-throughput | Precise environmental control; clean root harvesting | Risk of technical failure (e.g., pump failure) | Successful establishment in 4 weeks for cotton; used for heterogeneous nitrate supply studies [12] [13] |
Essential Research Reagent Solutions
The table below lists key materials required for establishing split-root systems, particularly in hydroponic contexts.
| Item | Function/Application | Protocol Example |
|---|---|---|
| Clone Collars | Support for suspending plants in hydroponic systems | Used as a sterile platform for cotton seedlings in a split-root hydroponic assay [12] |
| Agarose | Provides solid support for seed germination and seedling anchorage in compact hydroponic systems | 1% agarose solution used in PCR-tube-based Arabidopsis hydroponic system [13] |
| Nutrient Solutions (e.g., Long Ashton solution) | Provides essential macro and micronutrients in a controlled, soil-free environment | A modified Long Ashton solution used as a base for hydroponic split-root cultures [12] |
| Surface Sterilized Seeds | Ensures aseptic initiation of in vitro experiments, preventing microbial contamination | Seeds sterilized with bleach solution and rinsed with milli-Q water before germination [12] [13] |
| Air Pump & Air Stones | Oxygenates the nutrient solution in hydroponic reservoirs (e.g., DWC, some split-root setups) | Used in a nursery stage to promote root growth large enough for transplanting into SR-NFT systems [14] |
Experimental Workflow and Methodology
Detailed Protocol: Rapid Split-Root Assay in Hydroponics for Cotton
This protocol, validated on eight upland cotton varieties, establishes a split-root system within four weeks post-germination [12].
Workflow Diagram: Split-Root Establishment
Materials:
- Forceps, scissors, 250 mL glass beakers.
- Clone collars (e.g., Growneer, diameter 6.985 cm).
- Plastic planting tubs (e.g., Rubbermaid Pan, 11.4-Quart).
- Hydroponic nutrient solution (e.g., modified Long Ashton solution).
- Cable ties, sterilized with 90% ethanol.
Step-by-Step Method:
- Seed Sterilization and Germination: Surface-sterilize seeds using a 0.6% sodium hypochlorite (bleach) solution for 4 minutes, followed by three rinses with milli-Q water. Germinate seeds in a moist, sterile substrate like sand or a vermiculite/perlite mix [12].
- Seedling Growth: Grow seedlings until the first true leaves emerge (approximately 14 days after sowing) [12].
- Primary Root Excision: Carefully remove seedlings from the substrate and wash roots to remove all substrate. Using sterile scissors, cut off the primary root (taproot). This stimulates the growth of lateral roots [12].
- Hydroponic Transplant: Immediately transfer the seedling to a hydroponic system. Secure the plant using a sterile clone collar. The hydroponic solution promotes the rapid elongation of the lateral roots [12].
- Root System Division: Once the lateral roots are sufficiently long, gently divide them into two equal halves. Physically guide each half into a separate compartment of your split-root setup (e.g., two divided channels of an NFT system or two separate containers) [12] [14].
- Validation and Application: Before applying treatments, validate the uniformity of the split. The original protocol confirmed no statistically significant difference in root dry weight between the two halves across multiple cultivars. Once validated, each root half can be exposed to independent experimental treatments [12].
This guide provides a detailed protocol for establishing a split-root system in Arabidopsis thaliana using an optimized partial de-rooting method. The procedure is presented within the broader thesis context of enhancing the replicability and robustness of split-root assay research. A split-root system (SRS), where a plant's root system is divided into separate compartments, is a powerful tool for discerning local versus systemic regulation in plant responses to heterogeneous environments [4]. Robustness in experimental biology is defined as the capacity to generate similar outcomes despite slight variations in protocol, which is critical for efficient scientific progress and for ensuring research findings are relevant under variable natural conditions [1]. The optimized partial de-rooting method detailed here is designed to minimize plant stress, reduce recovery time, and improve survival rates compared to total de-rooting, thereby increasing the reliability and replicability of subsequent experimental data [4].
Optimized Partial De-Rooting Protocol
Materials and Reagents
Table 1: Research Reagent Solutions and Essential Materials
| Item Name | Function/Explanation |
|---|---|
| TK1 Medium (Optimized Arabidopsis Medium 1) | A growth medium with an NPK ratio of 5:1:3, optimal for in vitro Arabidopsis growth, promoting organized root meristems and protoplasts with regular ploidy [15]. |
| Bacto-Tryptone | Added in low concentration to TK1 medium to prevent formation of an insoluble pellet, ensuring all crucial nutrient elements remain available for plant growth [15]. |
| Plant Growth Agar | For solidifying culture media. |
| Sterile Surgical Scalpel/Blade | For performing the de-rooting procedure. A new blade is recommended for each experiment to minimize cell damage [15]. |
| Sterile Forceps | For handling seedlings during the procedure. |
| Square Petri Dishes (120 mm) | For growing seedlings and performing the initial de-rooting. |
Step-by-Step Procedure
- Plant Material Preparation: Germinate and grow Arabidopsis thaliana seeds on TK1 medium [15] in square Petri dishes under standard light and temperature conditions until the seedlings are 7 to 9 days old [4].
- Selection of Seedlings: Select healthy seedlings at the developmental stage where they possess a clear primary root and have developed at least two lateral roots.
- The Partial De-Rooting Procedure: Using a sterile surgical scalpel or razor blade, make a single clean cut approximately 0.5 cm below the shoot-to-root junction (the hypocotyl). This is the critical step that distinguishes the protocol from total de-rooting and leaves a portion of the main root attached to the shoot [4].
- Recovery Phase: Transfer the partially de-rooted seedlings to fresh TK1 medium. Allow the plants to recover and regenerate new lateral roots from the remaining root stub. The recovery time is significantly shorter than for totally de-rooted plants [4].
- SRS Establishment: Once the newly formed lateral roots are long enough (typically after a short recovery period), they can be carefully guided or transferred into separate physical compartments (e.g., two different agar plates or pots) to establish the split-root system.
Diagram 1: Partial vs. Total De-Rooting Workflow
Quantitative Comparison: Partial vs. Total De-Rooting
Table 2: Performance Metrics of De-Rooting Methods
| Metric | Partial De-Rooting (PDR) | Total De-Rooting (TDR) |
|---|---|---|
| Recovery Time | Significantly shorter [4] | Extended [4] |
| Survival Rate | Much higher [4] | Lower, especially at 9-11 DAS [4] |
| Final Rosette Area | Much closer to uncut plants [4] | Extremely decreased, particularly if de-rooting is performed past 10 DAS [4] |
| Recommended Time of Procedure | Less dramatic effect; viable at 11 and 15 DAS with slightly reduced final area [4] | Sharp decrease in final area if performed past 10 DAS [4] |
Troubleshooting Guide & FAQs
FAQ 1: Why does the protocol recommend partial de-rooting over total de-rooting?
Partial de-rooting is a less stressful procedure for the plant. By leaving a portion of the main root attached, the plant undergoes a shorter recovery time, achieves a higher survival rate, and develops a final rosette area that is much closer to that of uncut plants. This leads to more robust and reliable experimental subjects compared to the more severe stress induced by total de-rooting [4].
FAQ 2: What is the optimal developmental stage to perform the partial de-rooting procedure?
The procedure is most effective when performed on young seedlings, approximately 7 to 9 days after sowing (DAS). Performing the cut at this stage minimizes the impact on subsequent plant development. Delaying the procedure, particularly past 10 DAS in the case of total de-rooting, can drastically reduce the final rosette area [4].
FAQ 3: My plants are showing slow growth and poor survival after de-rooting. What could be the cause?
Poor outcomes are frequently linked to:
- Plant Developmental Stage: As noted above, performing the procedure too late can be detrimental [4].
- Sterility: Ensure all tools and surfaces are sterile to prevent infection.
- Tool Quality: Using a dull blade can cause excessive crushing and damage to the tissue. Always use a new, sharp scalpel or razor blade to make a clean cut [15].
- Growth Medium: The health of the donor plants is critical. Using an optimized growth medium like TK1 can improve the overall vigor of the plants and the quality of the starting material [15].
FAQ 4: How does this protocol contribute to the robustness of my split-root research?
This protocol directly addresses robustness by defining a method that is more tolerant to minor variations in execution. Because partially de-rooted plants are less stressed and recover faster, they are more likely to display consistent biological responses (e.g., preferential nutrient foraging) even if there are slight differences in lab conditions, researcher technique, or equipment. Investigating which protocol variations do or do not alter outcomes is key to robust research [1]. Using this optimized protocol reduces a major source of stress, thereby strengthening the validity of your findings.
FAQ 5: What molecular changes occur in the plant after de-rooting that I should be aware of?
The de-rooting procedure triggers distinct proteomic responses in the leaves. Total and partial de-rooting alter the plant's metabolic state during the healing process. This means that researchers must account for this "healing phase" in their experimental timeline and design, ensuring that the plants have fully recovered and stabilized before applying the experimental treatments (e.g., heterogeneous nutrient supply) in the split-root system [4].
Diagram 2: Stress & Recovery Signaling Post-De-Rooting
Frequently Asked Questions (FAQs)
FAQ 1: What is the key advantage of using a split-root system in plant biology research? The primary advantage is the ability to study systemic versus local plant responses. By physically separating the root system into two or more compartments that share a common shoot, researchers can apply different treatments (e.g., nutrients, pathogens, drought) to each section of the roots and observe how the plant coordinates its response across the entire organism [4] [1]. This is crucial for discerning true systemic signaling from local effects.
FAQ 2: My Arabidopsis plants have low survival rates after root splitting. What can I do? Research indicates that the partial de-rooting (PDR) method significantly improves survival and recovery compared to total de-rooting (TDR). Instead of cutting the root at the shoot-to-root junction, make the cut approximately half a centimeter below this junction, leaving a part of the main root attached. PDR leads to a shorter recovery time, a final rosette area closer to uncut plants, and a much higher survival rate [4].
FAQ 3: Why can't I replicate published split-root nutrient foraging phenotypes? Achieving replicability and robustness in split-root assays can be challenging due to extensive variations in protocols. Key factors to control and report include:
- Plant developmental stage at the time of cutting.
- Duration of the recovery period after splitting and before applying treatments.
- Precise concentrations of nutrients (e.g., high and low nitrate).
- Light levels, sucrose concentration in the media, and temperature [1]. Ensuring all these parameters are meticulously documented and consistent is vital for replicability.
FAQ 4: Can split-root systems be used to study interactions with soil microbes? Yes, this is a major application. Split-root systems are powerfully used to investigate whether plant-microbe interactions are governed by local or systemic mechanisms. For example, in legumes, they can determine how the plant systemically controls nodulation with rhizobial bacteria [16]. In woody plants like loblolly pine, they are used to study colonization strategies by ectomycorrhizal fungi [7] [17].
FAQ 5: How can I apply a water-soluble treatment to a drought-stressed plant without rehydrating it? The split-root system offers a solution. You can grow plants in a SRS with both halves subjected to water deprivation. The required water-soluble compound is applied to only one half of the root system. After the compound has been absorbed, that specific section of the root system can be excised from the main plant. This approach allows for the application of the compound while minimizing the rehydration effect and maintaining drought conditions [4].
Troubleshooting Guide
The table below outlines common experimental problems, their potential causes, and recommended solutions.
| Problem | Potential Causes | Recommended Solutions |
|---|---|---|
| Low Survival Rate | Excessively destructive de-rooting; incorrect developmental stage; microbial contamination [4]. | Use partial de-rooting (PDR); for Arabidopsis, perform cutting before 10 days after sowing (DAS); ensure sterile techniques [4]. |
| Poor Root System Development | Inadequate recovery time after splitting; suboptimal growth media [4] [10]. | Standardize and allow for a sufficient recovery period (e.g., 4-8 days) post-splitting before applying treatments; use validated hydroponic or agar media [1] [10]. |
| Lack of Robust/Replicable Results | Uncontrolled variations in protocol parameters; insufficient sample size; inconsistent root division [1]. | Meticulously document and standardize all protocol variables (light, media, timing); ensure root biomass is equally distributed between compartments [1] [10]. |
| Unsuccessful Nodulation Assays | Use of incompatible plant-rhizobia pairs; physical mixing of root compartments breaking isolation [18]. | Confirm symbiosis compatibility; use a physical barrier (e.g., twin-tube hydroponic system) to fully separate root halves and their microbial communities [18]. |
| Failed Drought Stress Application | Accidental rehydration via the treated root compartment; uneven root division leading to asymmetric stress [4]. | For compound application, remove the treated root half after absorption; confirm that both root compartments are of similar size and development stage at the start of drought [4]. |
Experimental Protocols for Key Applications
Protocol: Nutrient Foraging in Arabidopsis thaliana
This protocol is adapted from methodologies used to study systemic nitrate signaling [1].
Key Reagents:
- Growth Media: Agar plates containing a defined nitrogen source (e.g., 0.5 mM NH4+-succinate and 0.1 mM KNO3).
- Nitrogen Treatments: High Nitrate (HN): 5-10 mM KNO3; Low Nitrate (LN): 0.05-1 mM KNO3 or KCl as a control.
- Sucrose: 0.3% (w/v) in the media.
Methodology:
- Germination: Sow Arabidopsis seeds on agar plates and grow under long-day conditions (e.g., 16-h light/8-h dark) at 50-230 μmol mâ»Â² sâ»Â¹ light intensity and 22°C.
- Root Splitting (SNR method): At 7-10 days after sowing (DAS), cut the primary root tip to induce lateral root growth.
- Recovery: Allow plants to recover for 4-8 days for lateral roots to develop.
- Establish SRS: Select seedlings with two robust, similar-sized lateral roots. Carefully separate these two roots into two different compartments on a split-plate.
- Differential Treatment: Apply High Nitrate (HN) media to one compartment and Low Nitrate (LN) media to the other.
- Analysis: After 5-7 days, measure root growth parameters (e.g., lateral root length, density) in each compartment to quantify preferential foraging [1].
Protocol: Rhizobial Competitiveness in Soybean
This hydroponic protocol is used to discern local vs. systemic effects in nodulation [18].
Key Reagents:
- Plant Material: Soybean (Glycine max) seeds.
- Growth Medium: FÃ¥hraeus nutritive solution.
- Rhizobia Strains: e.g., Bradyrhizobium japonicum USDA110 and Sinorhizobium fredii HH103.
Methodology:
- Germination & Preparation: Surface-sterilize and germinate soybean seeds for two days. Excise the root tip and incubate for 120 hours in FÃ¥hraeus solution to promote lateral root growth.
- Seedling Selection: Select seedlings with two lateral roots of similar length and position. Remove all other lateral roots.
- Hydroponic SRS Setup: Transfer each selected seedling to a "twin-tube" system. Place one lateral root in each tube, separated by a physical barrier (e.g., filter paper against the tube wall).
- Inoculation: Inoculate the root halves differentially (e.g., one strain per half, or a mixture on one half and a control on the other).
- Monitoring: Monitor nodule development non-destructively over time (e.g., up to 34 days).
- Analysis: Record nodule number, fresh weight, and occupancy by different strains to assess local competitiveness [18].
Protocol: Drought Stress with Compound Application
This method allows for the application of compounds while maintaining drought stress [4].
Key Reagents:
- Water-Soluble Compound: The compound of interest to be tested under drought.
- Soil or Growth Substrate.
Methodology:
- Establish SRS: Using an appropriate method (e.g., PDR for Arabidopsis, SDR for larger plants), establish a plant with its root system split into two separate pots.
- Induce Drought: Subject both pots to water deprivation to induce drought stress.
- Compound Application: Apply the water-soluble compound dissolved in a small, minimal volume of solution to only one of the two droughted root compartments.
- Prevent Rehydration: Once the compound has been absorbed (determined empirically), carefully excise and remove the treated root half from the plant. This prevents that section from taking up more water and rehydrating the plant.
- Analysis: Proceed with physiological and molecular analyses to study the plant's response to the compound under maintained drought conditions [4].
The Scientist's Toolkit: Research Reagent Solutions
The table below lists essential materials and their functions for establishing and conducting split-root assays.
| Item | Function/Application |
|---|---|
| Agar Plates (with Sucrose) | Provides a transparent, controlled solid medium for growing young seedlings, ideal for root phenotyping and sterile work [1]. |
| Hydroponic Systems (Twin-Tube/Vessel) | Allows for non-destructive, continuous monitoring of root development and easy application of liquid treatments [18] [10]. |
| FÃ¥hraeus Nutritive Solution | A defined liquid medium specifically optimized for the growth of legumes and rhizobial cultures in symbiosis studies [18]. |
| High/Low Nitrogen Media | Key treatment for studying systemic nutrient signaling and root foraging behavior (e.g., 5 mM KNO3 vs. 5 mM KCl) [1]. |
| Rhizobial Strains (e.g., B. japonicum, S. fredii) | Nitrogen-fixing bacteria used as inoculants to study local and systemic regulation of nodulation in legumes [18] [16]. |
| Ectomycorrhizal Fungi (e.g., Paxillus spp.) | Symbiotic fungi used as inoculants to study colonization strategies and signaling in woody plants like pines [7] [17]. |
| Jzp-MA-11 | Jzp-MA-11, MF:C15H17FN4O2S, MW:336.4 g/mol |
| Egfr-IN-50 | Egfr-IN-50|Potent EGFR Kinase Inhibitor for Research |
Experimental Workflow and Signaling Pathways
Split-Root Assay Workflow for Systemic Response Analysis
Systemic Signaling in Nutrient Foraging and Nodulation
Troubleshooting Guides
Troubleshooting Split-Root Assay Replicability
| Problem | Potential Cause | Solution | Reference |
|---|---|---|---|
| Inconsistent root growth responses | Variation in high/low nitrate concentrations between labs. | Standardize nitrate concentrations (e.g., 5-10 mM KNO3 for HN; 0.05-1 mM KNO3 or KCl for LN). | [1] |
| Lack of robust preferential foraging phenotype | Insufficient recovery period after splitting the main root. | Implement a recovery period of 4-8 days on uniform nutrition before applying heterogeneous treatments. | [1] |
| High variability between experimental replicates | Differences in light intensity, photoperiod, or sucrose in media. | Control environmental factors: use long-day photoperiod, light intensity of 50-260 μmol mâ»Â² sâ»Â¹, and 0.3-1% sucrose as needed. | [1] |
| Unclear local vs. systemic signaling data | Inadequate separation of root halves or cross-contamination of solutions. | Use agar plate systems with physical barriers; ensure complete separation of root systems into distinct compartments. | [1] |
Troubleshooting Hydroponic Lettuce Pathogen Studies
| Problem | Potential Cause | Solution | Reference |
|---|---|---|---|
| Rapid spread of water-borne disease (e.g., Phytophthora cryptogea) | Recirculation of contaminated nutrient solution. | Implement system sterilization protocols; consider non-recirculating systems for infected plants; monitor pathogen levels. | [19] |
| Significant alterations in bacterial community upon pathogen infection | Pathogen-induced shift in microbiome masks treatment effects. | Include baseline microbiome profiling (e.g., 16S rRNA sequencing) of healthy plants as a control for every experiment. | [19] |
| Microbiome composition varies significantly between greenhouses | Context-dependent factors (water source, disinfection practices). | Design experiments with within-greenhouse replicates; do not pool samples from different facilities without controlling for location. | [19] |
| Indirect plant harm from microbial shifts (e.g., nanoplastics) | Tested compound alters microbial community, not plant directly. | Profile microbial species in irrigation water (e.g., Curvibacter fontanus abundance) in addition to plant health metrics. | [20] |
Frequently Asked Questions (FAQs)
FAQs on Split-Root Assays
Q: What is the minimum number of replicates required for a robust split-root assay? A: While three replicates are the absolute minimum, robust statistical analysis for higher-level research questions typically requires 12-18 total samples to confidently distinguish treatment effects from natural variability [21].
Q: How long should a typical split-root experiment with Arabidopsis last? A: Protocols vary, but a common timeline is: 7-13 days of growth before root cutting, a 4-8 day recovery period, and a 5-7 day heterogeneous treatment period [1].
Q: Why is my split-root assay not replicating published findings on systemic signaling? A: Small variations in protocol can significantly impact outcomes. Carefully standardize and report all parameters, including the concentrations of HN and LN treatments, light levels, sucrose concentration, and the duration of each growth stage [1].
FAQs on Hydroponic Lettuce-Microbiome Studies
Q: How do I sample the root microbiome of hydroponically cultivated lettuce? A: Sample as close to the rhizosphere (root zone) as possible, as this is the most biologically active region. For in-season sampling, pull soil cores from the root zone. Samples can be stored at room temperature if shipped within 5 days, or at -20°C (-4°F) for long-term storage [21].
Q: Can pathogen infection in hydroponic systems lead to a "cry-for-help" response in the lettuce microbiome? A: Yes. Some studies show that plants under stress, such as pathogen attack, can recruit beneficial microorganisms. However, this response is context-dependent. In some cases, infection leads to a decrease in beneficial microbes, so it is essential to profile the microbiome of both symptomatic and non-symptomatic plants [19].
Q: What are the critical parameters to monitor in a hydroponic nutrient solution? A: The two most critical parameters are Electrical Conductivity (EC), which measures nutrient concentration (ideal range: 1.5 to 3 dS mâ»Â¹), and acidity (pH), which should be maintained between 5.0 and 6.0 for optimal nutrient availability [22].
Split-Root Assay Protocol Variations
The table below summarizes key parameters from published split-root assays, highlighting the range of conditions used in successful studies.
| Publication | HN Concentration | LN Concentration | Light Intensity (μmol mâ»Â² sâ»Â¹) | Days Before Cutting | Recovery Period | Heterogeneous Treatment | Sucrose Concentration |
|---|---|---|---|---|---|---|---|
| Ruffel et al. (2011) | 5 mM KNO₃ | 5 mM KCl | 50 | 8-10 days | 8 days | 5 days | 0.3 mM |
| Remans et al. (2006) | 10 mM KNO₃ | 0.05 mM KNO₃ | 230 | 9 days | None | 5 days | None |
| Poitout et al. (2018) | 1 mM KNO₃ | 1 mM KCl | 260 | 10 days | 8 days | 5 days | 0.3 mM |
| Girin et al. (2010) | 10 mM NH₄NO₃ | 0.3 mM KNO₃ | 125 | 13 days | None | 7 days | 1% |
| Tabata et al. (2014) | 10 mM KNO₃ | 10 mM KCl | 40 | 7 days | 4 days | 5 days | 0.5% |
Essential Elements in Hydroponic Nutrient Solutions
The following table details the ionic forms and standard concentration ranges for essential elements in hydroponic nutrient solutions [22].
| Element | Ionic Form | Concentration Range (mg/L, ppm) |
|---|---|---|
| Nitrogen (N) | NO₃â», NH₄⺠| 100 to 200 |
| Phosphorus (P) | HPO₄²â», Hâ‚‚POâ‚„â» | 30 to 15 |
| Potassium (K) | K⺠| 100 to 200 |
| Calcium (Ca) | Ca²⺠| 200 to 300 |
| Magnesium (Mg) | Mg²⺠| 30 to 80 |
| Sulfur (S) | SO₄²⻠| 70 to 150 |
| Boron (B) | BO₃³⻠| 0.03 |
| Copper (Cu) | Cu²⺠| 0.01 to 0.10 |
| Iron (Fe) | Fe²âº, Fe³⺠| 2 to 12 |
| Manganese (Mn) | Mn²⺠| 0.5 to 2.0 |
Experimental Protocols
Detailed Protocol: Hydroponic System Setup for Lettuce
This protocol is adapted for laboratory research on lettuce-microbe interactions [23].
Key Materials:
- Lettuce seeds (Lactuca sativa L.)
- Nutrient solution components (see Table 3.2)
- Hydroponic containers and lids
- Foam panels or boards (e.g., styrofoam)
- Air pump and air stones (for Deep Water Culture)
- pH and EC meters
- Sterilization supplies (e.g., bleach, HCl)
Methodology:
- Seed Sterilization and Germination:
- Sterilize seeds using vapor-phase sterilization (4 hours in a desiccator with chlorine gas generated from 100 ml bleach and 3 ml HCl) or liquid sterilization methods [23].
- Place sterilized seeds on ¼ strength Murashige and Skoog (MS) media with vitamins, solidified with phytoagar.
- Stratify seeds in the cold room for two days, then transfer to a growth chamber (e.g., 23°C, 16h light/8h dark cycle). Seedlings will be ready for transplant in 10-12 days.
Nutrient Solution Preparation:
- Prepare a 10x concentrated stock solution of all macronutrients and micronutrients (except Fe-EDTA) in advance. Sterilize by autoclaving or filtration.
- Always add Fe-EDTA last when mixing the final nutrient solution to prevent precipitation.
- Bring the final solution to room temperature and adjust pH to 5.5-6.0 before use.
System Setup and Transplanting:
- Deep Water Culture (DWC) is recommended for its simplicity and suitability for root harvesting.
- Cut a foam panel to fit snugly inside your hydroponic container.
- Use a cork borer to create holes in the foam board, ensuring a density of approximately 1 plant per 10 cm² to prevent overcrowding [23].
- Insert foam tube plugs into the holes. These will hold the lettuce seedlings.
- Fill the reservoir with the prepared nutrient solution.
- Gently transplant seedlings from the agar plates into the foam plugs, ensuring the roots are submerged in the solution.
- Connect the air pump to the air stone(s) in the reservoir to provide oxygen to the roots.
System Maintenance:
- Monitor EC and pH daily and adjust as needed (see FAQ 2.2).
- Top off the reservoir with water to maintain volume. Completely replace the nutrient solution weekly.
- Maintain consistent climate conditions (temperature, humidity, light) as these directly impact water temperature and plant stress [24].
Detailed Protocol: Split-Root Assay for Nitrogen Foraging in Arabidopsis
This protocol outlines the key steps for investigating local and systemic signaling in response to heterogeneous nitrate supply [1].
Key Materials:
- Arabidopsis thaliana seeds
- Square Petri dishes
- Plant growth agar
- Nitrate sources (e.g., KNO₃)
- Control salts (e.g., KCl, Kâ‚‚SOâ‚„)
- Sterile surgical tools (scalpels, forceps)
Methodology:
- Plant Establishment:
- Surface-sterilize Arabidopsis seeds and sow on standard growth media containing a uniform, sufficient concentration of nitrate (e.g., 10 mM KNO₃).
- Stratify seeds and grow vertically in a growth chamber under controlled conditions (e.g., long-day photoperiod, 22°C) for 6-10 days until the primary root is well-established and two robust lateral roots have emerged.
Root Splitting:
- Under sterile conditions, use a scalpel to carefully remove the primary root tip just below the two lateral roots. This encourages the growth of the two lateral roots as the main root systems.
- Transfer the seedling to a split-root plate. The plate is divided into two compartments, each containing the same uniform nitrate media.
- Position the plant so that one lateral root is placed in each compartment.
- Allow the plants to recover and the lateral roots to establish for a 4-8 day "recovery period" [1].
Application of Heterogeneous Treatment:
- After the recovery period, this is the start of the experiment (T0).
- The treatment is applied by transferring the plant to a new split-root plate where one compartment contains High Nitrate (HN) media and the other contains Low Nitrate (LN) media.
- Ensure the root systems are fully separated with no cross-contamination between compartments.
Data Collection and Analysis:
- Grow plants under heterogeneous conditions for 5-7 days.
- Harvest root systems separately. Scan the roots from each compartment and use image analysis software (e.g, ImageJ) to quantify root architecture parameters, such as total root length, lateral root number, and root density for each side.
- Statistical analysis should account for the paired nature of the split-root system. A minimum of three biological replicates is required, with more replicates (12-18) providing greater confidence for robust statistical analysis [21].
Signaling Pathways and Workflows
Split-Root Assay Experimental Workflow
Lettuce-Pathogen-Microbiome Signaling
The Scientist's Toolkit: Research Reagent Solutions
| Item | Function / Application |
|---|---|
| Murashige and Skoog (MS) Basal Salt Mixture | A standardized blend of macro and micronutrients used for germinating seeds and as a base for plant growth media in controlled environments [23]. |
| KNO₃ / KCl for Nitrogen Treatments | Potassium nitrate serves as the standard source for High Nitrate (HN) treatments. Potassium chloride is often used as an osmotic control in Low Nitrate (LN) compartments in split-root assays [1]. |
| MES Hydrate | A pH buffering agent used in growth media to maintain a stable pH (typically 5.7) throughout the experiment, reducing variability [23]. |
| Propylene Monoazide (PMA) | A dye used to differentiate between DNA from live and dead microorganisms prior to DNA isolation, improving the accuracy of microbiome viability assessment [21]. |
| Fe-EDTA (Iron Chelate) | A soluble and bioavailable source of iron for plant nutrition in nutrient solutions. It is added last to prevent precipitation [23] [22]. |
| 16S rRNA Gene Primers | Universal primers used for amplicon sequencing to identify and profile the bacterial community composition in samples (e.g., rhizosphere, endosphere) [19]. |
| LysM Receptor Kinase Mutants | Key genetic tools in legume research to study the recognition of rhizobial Nod factors and investigate the overlap between symbiosis and pathogen defense pathways [25]. |
| Cys Cluster Proteins (CCPs) | Nodule-specific proteins with antimicrobial activity being investigated for their role in protecting legume nodules from pathogens [25]. |
| D-Iditol-13C | D-Iditol-13C, MF:C6H14O6, MW:183.16 g/mol |
Solving the Replicability Crisis: A Troubleshooting Guide for Common Split-Root Assay Challenges
Troubleshooting Guides
Guide 1: Poor Plant Survival After Establishing a Split-Root System
Problem: Low survival rates of plants following the de-rooting procedure required to create a split-root system (SRS).
Explanation: Total de-rooting is a highly stressful event for a plant. The removal of the entire root system severely disrupts water and nutrient uptake and depletes energy reserves, leading to high mortality.
Solution:
- Implement Partial De-Rooting: Instead of cutting the root at the shoot-to-root junction (total de-rooting), make the cut approximately half a centimeter below this junction, leaving a portion of the main root attached [26].
- Justification: Studies on Arabidopsis thaliana show that partial de-rooting is a less stressful procedure. Plants recover more quickly and show a final rosette area much closer to that of uncut plants compared to totally de-rooted plants [26].
- Verify Plant Vigor: Ensure that plants selected for the procedure are healthy and have adequate carbohydrate reserves to support recovery and new root growth [27].
Guide 2: Inconsistent Results in Split-Root Experiments
Problem: Difficulty achieving replicable and robust results between different experimental runs or personnel.
Explanation: The complexity of split-root assays allows for extensive variation in protocols (e.g., timing, growth conditions, exact cutting method). Small, undocumented differences can lead to different outcomes, affecting the robustness of your research [28] [29].
Solution:
- Document Protocol Variations Meticulously: Extend the level of detail in your research protocols. Record and report not just the main steps, but also seemingly minor details such as the precise age of the seedlings at the time of cutting, light levels, and nutrient concentrations [28].
- Validate Key Steps: Investigate which aspects of your protocol are essential for the desired outcome. For example, determine the optimal recovery period after root washing if exudates are being collected, as a recovery period of at least 3 days is critical to prevent bias from root damage [30].
- Standardize the De-Rooting Method: Across a project, consistently use the partial de-rooting method for young Arabidopsis seedlings to minimize variability introduced by differing stress levels from the procedure [26].
Frequently Asked Questions (FAQs)
FAQ 1: What is the fundamental difference between partial and total de-rooting?
Answer: The difference lies in the amount of root tissue removed.
- Total De-Rooting (TDR): The main root is severed at the shoot-to-root junction, removing the entire root system [26].
- Partial De-Rooting (PDR): The main root is cut approximately 0.5 cm below the shoot-to-root junction, leaving a stub of the primary root attached to the shoot [26].
FAQ 2: Why does a partial de-rooting procedure lead to faster recovery?
Answer: The stub of the main root left in PDR plants likely serves as an energy reservoir and may contain meristematic tissue that facilitates faster regeneration of new lateral roots. Proteomic analyses indicate that partially and totally de-rooted plants undergo distinct metabolic alterations during the healing process, with PDR plants experiencing less severe stress [26].
FAQ 3: My research involves applying a compound to drought-stressed plants. Can a split-root system help?
Answer: Yes. A split-root system is an excellent tool for drought experiments involving water-soluble compounds. You can grow plants in an SRS with both halves subjected to water deprivation. The required compound can be applied to one half of the root system. Once absorbed, that specific half can be excised from the main plant, thereby minimizing rehydration of the plant and maintaining the overall drought conditions [26].
FAQ 4: Beyond survival, how does de-rooting stress affect my plants?
Answer: Severe root removal induces significant physiological stress. Immediately after root pruning, plants can experience water stress, reduced photosynthesis, and transpiration. Shoot growth may be temporarily reduced as the plant re-allocates photosynthates to support the regeneration of new roots and re-establish root-shoot balance [27].
Data Presentation: Partial vs. Total De-Rooting
The following table summarizes quantitative data comparing the two de-rooting methods in Arabidopsis thaliana.
Table 1: Comparative Analysis of De-Rooting Techniques on Plant Performance
| Performance Metric | Partial De-Rooting (PDR) | Total De-Rooting (TDR) | Reference |
|---|---|---|---|
| Recovery Time | Shorter (e.g., 7.4 - 7.6 days) | Longer (e.g., 8.5 days) | [26] |
| Final Rosette Area | Much closer to uncut plants | Significantly smaller than uncut plants | [26] |
| Survival Rate | Higher (e.g., 88% at 4 DAS*) | Lower (e.g., 73% at 6 DAS*) | [26] |
| Root System Development | More developed root system | Less developed root system | [26] |
| Physiological Stress | Less stressful procedure; distinct, less severe proteomic alterations | Highly stressful procedure; distinct, severe proteomic alterations | [26] |
*DAS: Days After Sowing
Experimental Protocols
Protocol 1: Establishing a Split-Root System via Partial De-Rooting
This protocol is optimized for young Arabidopsis thaliana seedlings to minimize stress and improve experimental robustness [26].
Key Materials:
- Sterilized seeds of Arabidopsis thaliana
- In vitro growth media or soil
- Sterile surgical blades or fine scissors
- Laminar flow hood (for in vitro work)
- Split-root containers (e.g., divided plates or pots)
Methodology:
- Germination and Pre-growth: Germinate seeds and grow plantlets under standard conditions until they develop a primary root and the first two lateral roots begin to emerge.
- Partial De-Rooting: Using a sterile blade, carefully cut the primary root approximately 0.5 cm below the shoot-to-root junction. Avoid cutting at the junction itself.
- Recovery Phase: Transfer the de-rooted seedlings to a fresh plate or pot with growth media. Allow the plants to recover and develop new lateral roots from the remaining root stub. Monitor for regained growth rates.
- Splitting the Roots: Once two new lateral roots are sufficiently long, gently guide each root into two separate compartments of your split-root system. These compartments will later be used for differential treatments.
- Acclimatization and Treatment: Allow the root systems to establish in their respective compartments before applying any experimental treatments.
Protocol 2: Ensuring Robustness in Split-Root Assays
This is a framework for enhancing the reliability of your split-root experiments, based on an analysis of methodological variations [28] [29].
Key Principle: Systematically document and control variables to determine which are essential for your specific research outcome.
Methodology:
- Define Core and Variable Parameters: List every step of your protocol. Categorize parameters into "core" (must not change, e.g., genetic background) and "variable" (to be tested for robustness, e.g., nitrogen concentration, photoperiod, recovery time).
- Test Key Variables: If possible, run small-scale pilot experiments to see how variations in key parameters (like those in the table below) affect your primary outcome (e.g., preferential root foraging).
- Detailed Documentation: For every experiment, record all parameters in extreme detail. This includes seed lot numbers, exact ages of plants, time of day for procedures, and environmental conditions.
- Replicate Appropriately: Include sufficient biological and technical replicates within and across experimental runs to account for inherent noise.
Table 2: Key Parameters to Document for Robust Split-Root Assays
| Category | Parameter Examples | Importance for Robustness |
|---|---|---|
| Plant Material | Species, ecotype/cultivar, seed age, germination rate | Genetic and physiological baseline [28] |
| Growth Conditions | Light intensity & photoperiod, temperature, humidity, media type (agar/soil) | Defines the pre-treatment environment [28] |
| De-Rooting Procedure | Exact cutting location (PDR/TDR), seedling age (DAS), tool sterilization | Directly impacts survival and recovery time [26] |
| Nutrient Treatment | Concentrations of high/low nitrate/nutrient, duration of treatment, pH of media | Central to the experimental question [28] |
| Recovery & Timing | Duration of recovery after de-rooting, duration of treatment before harvest | Affects plant stress levels and response magnitude [30] |
Signaling and Workflow Visualization
Split-Root Establishment and Recovery Workflow
Plant Systemic Signaling Post-Root Damage
The Scientist's Toolkit: Research Reagent Solutions
Table 3: Essential Materials for Split-Root and Root Stress Research
| Item | Function/Application | Specific Example/Note |
|---|---|---|
| Sterile Surgical Blades | For precise partial or total de-rooting cuts. | Ensure sharpness to minimize crushing tissue. |
| Split-Containers | To physically separate root halves for differential treatment. | Divided agar plates; stacked pots; net pots [26] [28]. |
| Plant Growth Media | Support plant growth in controlled conditions. | Agar-based media for in vitro work; standardized soil mixes. |
| Phytohormones (e.g., Auxins) | Study signaling pathways or induce rooting. | Indole-3-butyric acid (IBA) used in rooting studies [31]. |
| Rhizogenic Bacterium | Alternative, natural rooting agent. | Rhizobium rhizogenes can induce hairy root formation [31]. |
| Proteomics Kits | Analyze metabolic alterations in response to stress. | Used to identify stress-specific protein changes in leaves [26]. |
Troubleshooting Guide: Addressing Common Split-Root Assay Challenges
This guide addresses frequent issues researchers encounter when establishing and interpreting split-root assays, focusing on how protocol variations impact experimental robustness.
FAQ: Systemic Signaling and Preferential Foraging
Q: My split-root assay fails to show the expected preferential root growth in high nitrate. What could be wrong?
- A: The heterogeneous nitrate response is systemic. Ensure your split is clean, with each half fully isolated in its compartment. Verify the health of the plants post-splitting; a difficult recovery can mask subtle growth responses. The method of de-rooting (partial vs. total) can significantly affect recovery time and subsequent health, influencing the plant's ability to mount a systemic response [4].
Q: Why do I get inconsistent root architecture measurements between experimental replicates?
- A: Inconsistencies often stem from variations in pre-growth conditions. As shown in Table 1, factors like the number of days before root cutting, the duration of the recovery period, and light intensity vary widely across protocols. Strictly standardizing these variables within your lab is crucial for replicability [1].
Q: My control plants (homogeneous nutrient conditions) show different growth than my untreated, non-split plants. Is this normal?
- A: Yes. The physical process of creating a split-root system is a stressor. Proteomic analyses show that the de-rooting procedure itself triggers distinct metabolic alterations in the leaves. Therefore, comparing treatment groups against appropriate split-root controls (e.g., HNHN or LNLN) is more valid than comparing them to unsplit plants [4].
Troubleshooting Table: Protocol Variables and Solutions
| Symptom | Potential Cause | Recommended Solution |
|---|---|---|
| Low survival rate after splitting | Severe de-rooting stress | Use partial de-rooting (leaving part of the main root) instead of total de-rooting for younger plants to minimize stress and shorten recovery [4]. |
| High variability in root growth responses between plants | Inconsistent light intensity or photoperiod | Standardize light conditions. Refer to Table 1; protocols use intensities from 40 to 260 mmol mâ»Â² sâ»Â¹ and different photoperiods (long or short day) [1]. |
| Weak or absent preferential foraging signal | Incorrect nitrate concentrations or source | Review and adjust high/low nitrogen concentrations and ensure ionic strength is balanced with salts like KCl or Kâ‚‚SOâ‚„ (see Table 1 for standard ranges) [1]. |
| Altered plant development, confounding results | Presence/absence of sucrose in media | Decide and standardize sucrose use. Different protocols use 0%, 0.3 mM, 0.5%, or 1% sucrose, which can affect plant metabolism and development [1]. |
| Extended recovery time, delayed experiments | Insufficient recovery period after splitting | Allow adequate recovery time for plants to regain normal growth rates before applying experimental treatments. This can range from 0 to 8 days depending on the protocol and plant age [4] [1]. |
Experimental Protocols: Methodologies for Robust Split-Root Assays
Detailed Protocol: Establishing a Split-Root System in Arabidopsis
The following methodology is optimized for young Arabidopsis seedlings, emphasizing minimal stress [4].
Pre-growth and Germination:
- Surface-sterilize Arabidopsis seeds and sow them on a standard nutrient agar medium.
- Stratify seeds at 4°C for 2-4 days to synchronize germination.
- Transfer plants to a growth chamber with controlled conditions (e.g., 22°C, long-day photoperiod with specific light intensity).
De-rooting and Splitting Procedure:
- Timing: Perform the procedure when seedlings are 8-10 days old, after the development of two lateral roots.
- Technique (Partial De-rooting):
- Using a sterile scalpel, make a clean cut on the primary root approximately 0.5 cm below the shoot-to-root junction. This preserves a portion of the primary root.
- Rationale: Partial de-rooting results in a significantly shorter recovery time, a higher survival rate, and a final rosette area closer to that of uncut plants compared to total de-rooting [4].
- Transfer the de-rooted seedlings to fresh media to encourage the growth of the two lateral roots.
Recovery and System Establishment:
- Allow the plants to recover and develop two robust lateral root systems. This recovery period is critical and should be standardized (e.g., 4-8 days).
- Once the lateral roots are long enough, carefully transfer the plant to the split-root setup (e.g., a divided agar plate or a twin-pot system), placing one root in each compartment.
Application of Heterogeneous Treatments:
- After the root systems are established in their separate compartments, apply the differential treatments (e.g., high nitrate vs. low nitrate).
- Maintain treatments for a defined period, typically 5-7 days, before harvesting and analyzing root growth.
Visualizing the Split-Root Workflow and Signaling
The following diagram illustrates the key experimental steps and the systemic signaling concept investigated with this method.
Quantitative Data: Comparing Published Split-Root Protocols
A review of literature reveals significant variation in split-root protocols for nitrate foraging studies. Understanding this variation is key to troubleshooting and ensuring robust, replicable results [1].
Table: Variations in Split-Root Assay Protocols for Nitrate Foraging
| Publication | HN Concentration | LN Concentration | Photoperiod & Light Intensity (μmol mâ»Â² sâ»Â¹) | Days Before Cutting | Recovery Period | Sucrose in Media |
|---|---|---|---|---|---|---|
| Ruffel et al. (2011) | 5 mM KNO₃ | 5 mM KCl | Long day - 50 | 8-10 days | 8 days | 0.3 mM |
| Remans et al. (2006) | 10 mM KNO₃ | 0.05 mM KNO₃ | Long day - 230 | 9 days | None | None |
| Poitout et al. (2018) | 1 mM KNO₃ | 1 mM KCl | Short day - 260 | 10 days | 8 days | 0.3 mM |
| Girin et al. (2010) | 10 mM NH₄NO₃ | 0.3 mM KNO₃ | Long day - 125 | 13 days | None | 1% |
| Tabata et al. (2014) | 10 mM KNO₃ | 10 mM KCl | Long day - 40 | 7 days | 4 days | 0.5% |
The Scientist's Toolkit: Essential Research Reagent Solutions
This table details key materials and their functions for establishing successful split-root assays.
Table: Key Reagents and Materials for Split-Root Assays
| Item | Function / Rationale |
|---|---|
| Agar Plates (Divided) | A common growth system for young seedlings; allows for clear physical separation of root halves and easy visualization of root architecture [4] [1]. |
| Type B (High-Purity) Silica Columns | For HPLC analysis of metabolites; recommended to avoid peak tailing of basic compounds caused by interactions with silanol groups in lower-purity silica [32]. |
| KNO₃ / KCl / K₂SO₄ | Standard salts used to create defined High Nitrogen (HN) and Low Nitrogen (LN) conditions while balancing ionic strength and potassium levels across treatments [1]. |
| Viper or nanoViper Fingertight Fitting Capillaries | HPLC capillaries with minimal inner diameter (e.g., 0.13 mm for UHPLC) to reduce extra-column volume, which can cause peak broadening and tailing [32]. |
| Sucrose | An optional carbon source in growth media. Its presence (0-1%) and concentration must be standardized as it influences plant metabolic status and development [1]. |
Frequently Asked Questions
What is the single most impactful step I can take to minimize surgical artifacts in my split-root assay? Adopt the partial de-rooting (PDR) method over total de-rooting (TDR). Research shows that leaving a small portion (approx. 0.5 cm) of the main root attached during the splitting procedure significantly reduces recovery time, increases survival rates, and results in a final rosette area much closer to that of uncut plants, indicating lower systemic stress [4].
My negative control plants (both sides in low nitrate) show unexpected root growth variations. Could this be a surgical artifact? Yes. The surgical procedure itself can create inherent variability. The stress from de-rooting and recovery can affect the plant's subsequent response to nutrient conditions. It is crucial to include the appropriate control plants that have undergone the exact same surgical procedure but are placed in homogeneous nutrient conditions (e.g., LNLN) for a valid baseline comparison [1].
How long should I allow for plant recovery after the split-root surgery before applying my experimental treatment? The recovery period is protocol-dependent and critical for robustness. While some methods use no recovery period, others employ a defined healing phase of 3 to 8 days before applying heterogeneous treatments [1]. You should determine the recovery time by monitoring plants until they regain relative growth rates equal to uncut control plants [4].
Beyond root growth, what other plant systems might be affected by the surgical stress? The stress from the procedure can induce widespread metabolic alterations. A proteomic analysis of Arabidopsis leaves following de-rooting revealed distinct metabolic changes in partially and totally de-rooted plants during the healing process. This suggests the surgical artifact can affect the entire plant's physiology, which must be considered when interpreting data from any tissue [4].
Are surgical artifacts a significant concern in woody plant species as well? Yes. The physical damage from root pruning or splitting in woody plants can make them more susceptible to pathogen infection and induce plant defense responses, creating artifacts that confound the experimental results [7].
Troubleshooting Guide
| Symptom | Possible Cause | Solution |
|---|---|---|
| Low survival rate after splitting | Excessive surgical stress from total de-rooting (TDR); incorrect developmental stage [4]. | Switch to partial de-rooting (PDR). For Arabidopsis, avoid de-rooting at the sensitive four-leaf stage where hypocotyl contact with media is difficult [4]. |
| High variability in root foraging responses | Inconsistent recovery periods or unaccounted-for protocol variations between experiment batches [1]. | Standardize and document all recovery times, light levels, and media components. Use the table below to benchmark your protocol against established ones [1]. |
| Poor root system development on one or both sides | Physical damage to the emerging lateral roots during the splitting or transfer process [4]. | Refine surgical technique under a microscope. Ensure both root compartments have equal access to moisture and are not physically constrained. |
| Confounding results in systemic signaling studies | Failure to include proper surgical controls. The systemic signal could be a stress response to the cut, not the localized treatment [4] [7]. | Always include control plants that have undergone the split-root surgery but are placed in homogeneous conditions for both root halves. |
Quantitative Data for Experimental Planning
The table below summarizes key growth parameters from a systematic study comparing de-rooting techniques in Arabidopsis, providing benchmarks for expected outcomes and artifacts.
Table 1: Impact of De-rooting Method on Plant Development [4]
| De-rooting Method | Time of De-rooting (Days After Sow) | Recovery Time (Days) | Final Rosette Area (cm²) | Survival Rate (%) |
|---|---|---|---|---|
| Partial De-rooting (PDR) | 7 | ~5 | ~4.5 | ~95 |
| Partial De-rooting (PDR) | 11 | ~5 | ~3.5 | ~90 |
| Total De-rooting (TDR) | 7 | >7 | ~2.5 | ~80 |
| Total De-rooting (TDR) | 11 | >10 | ~1.5 | ~70 |
| Uncut Control | N/A | N/A | ~5.0 | ~100 |
Experimental Protocol Variations and Robustness
Different published protocols for split-root assays in Arabidopsis nitrate foraging studies show significant variation. When replicating or designing experiments, note that while the primary finding of preferential foraging is robust, secondary phenotypes may be more sensitive to these protocol details.
Table 2: Protocol Variations in Arabidopsis Split-Root Nitrate Foraging Assays [1]
| Publication | HN Concentration | LN Concentration | Days Before Cutting | Recovery Period | Sucrose in Media |
|---|---|---|---|---|---|
| Ruffel et al. (2011) | 5 mM KNO₃ | 5 mM KCl | 8-10 | 8 days | 0.3% |
| Remans et al. (2006) | 10 mM KNO₃ | 0.05 mM KNO₃ | 9 | None | None |
| Poitout et al. (2018) | 1 mM KNO₃ | 1 mM KCl | 10 | 8 days | 0.3% |
| Tabata et al. (2014) | 10 mM KNO₃ | 10 mM KCl | 7 | 4 days | 0.5% |
The Scientist's Toolkit: Essential Research Reagents and Materials
Table 3: Key Materials for Split-Root Assays
| Item | Function / Application |
|---|---|
| Arabidopsis thaliana | A common model organism with a well-characterized root system, ideal for developing and optimizing split-root protocols [4] [1]. |
| Partial De-rooting Protocol | A surgical method to minimize artifacts by leaving a segment of the primary root intact, reducing stress and improving survival and growth consistency [4]. |
| Homogeneous Nutrient Controls | Essential control plants that have undergone the split-root surgery but have both halves in the same nutrient condition (e.g., High-High or Low-Low) to account for systemic effects of the procedure itself [1]. |
| Agar-Based Growth Media | Allows for precise control of nutrient composition and physical support for root growth in vitro, facilitating the split-root setup [4] [1]. |
| Nitrate Isotopes (e.g., ¹âµN) | Used to trace ion uptake and transport mechanisms between the differentially treated root halves, providing direct evidence of systemic regulation [7]. |
Decision Workflow for Split-Root Experimental Design
The following diagram outlines key decision points to minimize surgical artifacts when planning a split-root experiment.
Actionable Recommendations for Enhanced Protocol Documentation and Reporting
Troubleshooting Common Split-Root Assay Challenges
FAQ: How can I minimize plant stress during split-root establishment?
Problem: High seedling mortality or stunted growth after root division.
- Recommendation A: Implement Partial De-rooting: For species like Arabidopsis, perform a partial cut approximately 0.5 cm below the shoot-to-root junction instead of complete removal. This method significantly shortens recovery time, improves survival rates, and results in final rosette area much closer to uncut plants compared to total de-rooting [4].
- Recommendation B: Optimize Developmental Timing: The plant's developmental stage at cutting critically impacts success. For Arabidopsis, performing the procedure at 11 or 15 days after sowing (DAS) on totally de-rooted plants sharply decreased final leaf area and extended recovery time. Partial de-rooting showed less sensitivity to timing [4].
- Recommendation C: Ensure Medium Contact: For totally de-rooted plants at the four-leaf-stage, ensure the hypocotyl maintains contact with the growth medium. Plants with all leaves of similar size often fail to maintain contact, leading to desiccation or falling [4].
FAQ: How do I validate successful compartment separation in my system?
Problem: Uncertainty about whether root compartments remain truly isolated during treatments.
- Validation Method A: Biomass Comparison: Quantify root dry weight from both halves of the root system after establishment. Statistical comparison (e.g., Kruskal-Wallis and Wilcoxon signed-rank tests) should show no significant difference between sides under control conditions, confirming equal division [10].
- Validation Method B: Tracer Application: Apply a visible dye, fluorescent marker, or treatment with localized effects to one compartment only. Confirm the absence of the tracer in the opposite compartment and check for expected localized physiological responses [17].
- Validation Method C: Microbial Confinement Testing: When studying root-microbe interactions, inoculate one side with mycorrhizal fungi or other microorganisms and confirm their absence on the non-inoculated side through microscopic examination or molecular detection [17].
FAQ: Why do I get inconsistent results when replicating published split-root protocols?
Problem: Difficulty achieving consistent experimental outcomes across laboratories or personnel.
- Solution A: Document Critical Protocol Variables: Explicitly record and control key parameters that significantly impact outcomes. Research shows variations in nitrogen concentrations, light intensity, photoperiod, sucrose concentration, and recovery periods exist across published protocols [1].
- Solution B: Establish Internal Replicability Standards: Implement the ALCOA+ framework for documentation: Ensure data is Attributable, Legible, Contemporaneous, Original, and Accurate, plus Complete, Consistent, Enduring, and Available [33] [34].
- Solution C: Conduct Robustness Testing: Systematically test which protocol variations substantially affect outcomes and which are buffered against. Outcomes robust to slight variations are more likely relevant under natural conditions and transferable between labs [1].
Quantitative Data Comparison for Split-Root Systems
Table 1: Split-Root Establishment Parameters Across Plant Species
| Species | Method | Time to Establish | Key Validation Measurement | Reference |
|---|---|---|---|---|
| Upland Cotton (8 varieties) | Primary root cut + hydroponics | 4 weeks post-germination | Root dry weight (no significant difference between halves) | [10] |
| Loblolly Pine | Primary root tip cut + hydroponics | 8 weeks post-germination | Root biomass comparison; absence of cross-compartment colonization | [17] |
| Arabidopsis thaliana | Partial de-rooting (0.5 cm below junction) | Shorter recovery vs. total de-rooting | Rosette area, survival rate, proteomic analysis | [4] |
Table 2: Protocol Variations in Arabidopsis Split-Root Nitrate Foraging Studies
| Study Parameter | Ruffel et al. | Remans et al. | Poitout et al. | Girin et al. |
|---|---|---|---|---|
| HN Concentration | 5 mM KNO₃ | 10 mM KNO₃ | 1 mM KNO₃ | 10 mM NH₄NO₃ |
| LN Concentration | 5 mM KCl | 0.05 mM KNO₃ | 1 mM KCl | 0.3 mM KNO₃ |
| Days Before Cutting | 8-10 days | 9 days | 10 days | 13 days |
| Recovery Period | 8 days | None | 8 days | None |
| Sucrose Concentration | 0.3 mM | None | 0.3 mM | 1% |
| Light Intensity | 50 μmol mâ»Â² sâ»Â¹ | 230 μmol mâ»Â² sâ»Â¹ | 260 μmol mâ»Â² sâ»Â¹ | 125 μmol mâ»Â² sâ»Â¹ |
Detailed Experimental Methodologies
Standardized Hydroponic Split-Root Protocol for Cotton
This methodology enables reliable split-root establishment across multiple cotton varieties within four weeks post-germination [10]:
- Germination: Germinate cotton seeds under standard conditions until radicle emergence.
- Primary Root Excision: Carefully cut the primary root of seedlings.
- Hydroponic Transfer: Immediately transplant prepared seedlings into hydroponic system.
- Lateral Root Promotion: Maintain in hydroponic conditions to stimulate uniform lateral root growth.
- Root Division: Once lateral roots reach sufficient length, physically separate them into two equal compartments.
- Experimental Application: Apply independent treatments to each root compartment while maintaining shared aerial environment.
- Validation: Harvest and measure root dry weight from both compartments to confirm equal distribution.
Partial vs. Total De-rooting Methodology for Arabidopsis
This protocol minimizes stress for small plants like Arabidopsis, enabling earlier experimentation [4]:
- Plant Growth: Grow Arabidopsis seedlings on appropriate medium under controlled conditions.
- Surgical Procedure:
- Partial De-rooting (Recommended): Cut main root approximately 0.5 cm below shoot-to-root junction.
- Total De-rooting (Alternative): Cut root directly at shoot-to-root junction (less favorable).
- Recovery Monitoring: Track time to regain normal growth rates relative to uncut plants.
- Root Development: Allow new lateral roots to develop from remaining tissue.
- Compartment Separation: Guide emerging lateral roots into separate physical compartments.
- System Validation: Compare survival rates, recovery time, final rosette area, and root system development between methods.
Signaling Pathways and Experimental Workflows
Split-Root Assay Establishment Workflow
Systemic Signaling in Split-Root Nitrogen Foraging
Research Reagent Solutions and Essential Materials
Table 3: Essential Research Materials for Split-Root Assays
| Material/Reagent | Function/Application | Example Specifications |
|---|---|---|
| Hydroponic Growth Systems | Promotes rapid lateral root elongation after primary root cutting | Used for cotton [10] and pine [17] protocols |
| Agar Plates with Dividers | Physical separation of root compartments for small plants | Used for Arabidopsis studies [1] [4] |
| Nitrate Sources (KNO₃, NH₄NO₃) | High/low nitrate treatments for nutrient foraging studies | Concentrations vary: 1-10 mM for HN; 0.05-1 mM for LN [1] |
| Sucrose Supplement | Carbon source for in vitro growth | Varies by protocol: 0-1% in media [1] |
| Ectomycorrhizal Fungi | Microbial inoculation studies for systemic signaling | e.g., Paxillus ammoniavirescens, Hebeloma cylindrosporum [17] |
Ensuring Data Integrity: Validation Strategies and Comparative Analysis of Split-Root Outcomes
For researchers investigating root systems, precise benchmarking of growth parameters is fundamental for obtaining reliable and interpretable data. This is especially critical in complex experimental setups like split-root assays, where the goal is to unravel local and systemic signaling in plant responses to heterogeneous nutrient environments. Achieving robustness—the capacity to generate similar outcomes despite slight variations in experimental protocol—is a key indicator of a significant biological phenomenon and is essential for research that can be built upon by other labs, potentially using different equipment or conditions [1]. This guide addresses the common challenges in benchmarking root architecture and provides targeted troubleshooting advice to enhance the replicability and robustness of your research.
FAQs & Troubleshooting Guides
FAQ 1: What is the difference between reproducibility, replicability, and robustness?
- Reproducibility typically refers to the ability to generate quantitatively identical results when using the exact same methods, data, and computational code [1].
- Replicability describes the ability of an experiment, performed under the same conditions (potentially in a different lab or by a different researcher), to produce quantitatively and statistically similar results. In experimental biology, perfect reproducibility is often unattainable due to biological noise, so replicability is the more common and practical goal [1].
- Robustness refers to the capacity of an experimental outcome to remain consistent in the face of slight variations in the protocol, such as changes in nutrient concentrations, light levels, or the duration of a treatment. A robust result is more likely to be biologically relevant and is a key objective for optimizing split-root assay reliability [1].
FAQ 2: Why do my root architecture trait measurements differ from published results, even when using the same species?
This is a frequent challenge, often stemming from three main sources:
- Interspecific and Intraspecific Variation: Root traits exhibit natural variation both between different species (interspecific) and among individuals of the same species (intraspecific). For example, a study on 47 annual ephemeral species found that the variation in traits like Root Tissue Density (RTD) originated significantly from both interspecific (48.78%–99.76%) and intraspecific (contribution to RTD variation was 51.22%) sources [35]. Always account for this natural variation in your experimental design and analysis.
- Variation Across Root Orders: The classification of fine roots by their order (e.g., first-order, second-order) is critical. Lower-order roots often align with resource acquisition strategies, while higher-order roots are more conservative. Analyzing a mix of root orders without distinction can lead to inconsistent and misleading trait measurements [36].
- Inherent Protocol Diversity: Published protocols for even standardized assays like split-root experiments can vary widely. A review of Arabidopsis split-root assays revealed extensive differences in nitrate concentrations, photoperiods, sucrose levels, and recovery periods across studies [1]. These variations can directly impact the observed root architecture.
Troubleshooting Guide: Unexplained Variation in Root Trait Measurements
| Symptom | Potential Cause | Solution |
|---|---|---|
| Inconsistent measurements of Specific Root Length (SRL) or Root Tissue Density (RTD). | High intraspecific variation or mixing of different root orders during analysis. | Increase biological replicates and implement a root order classification system during sampling [35] [36]. |
| Inability to replicate a published phenotypic response in a split-root assay. | Undocumented critical step or parameter in the published protocol. | Systematically test key protocol variables (e.g., growth media sucrose concentration, exact duration of recovery period) to determine essential parameters for robustness [1]. |
| High error in estimated root length or branch count from image analysis. | Overlapping or crossing root segments in complex architectures, leading to software analysis errors [37]. | Use validation tools like ArchiSimple to create synthetic root images with known "ground-truth" data to calibrate your image analysis pipeline [37]. |
| Root system architecture (RSA) model fails to match experimental observations. | Poorly constrained model parameters or an incorrect model structure. | Employ an Approximate Bayesian Computation (ABC) framework to infer the most probable mechanistic parameters and compare model structures against your data [38]. |
FAQ 3: How can I validate the accuracy of my root image analysis pipeline?
Many automated root image analysis tools are not thoroughly validated on large, complex root systems, which can lead to underestimated biases [37].
- Best Practice: Generate a library of synthetic root system images using a structural model like ArchiSimple. This provides perfect "ground-truth" data for traits like total root length and branch count. You can then process these synthetic images through your analysis pipeline to evaluate its accuracy and identify systematic errors [37].
- Alternative Approach: For model-based analyses, use Approximate Bayesian Computation (ABC). This likelihood-free inference method allows you to calibrate model parameters and select the most appropriate model structure by comparing simulated outputs to your observed root architectures, thereby quantifying uncertainty [38].
FAQ 4: What are the best practices for ensuring my split-root assay results are robust?
The complexity of multi-step split-root assays makes them particularly prone to replicability issues [1].
- Document Protocol Variations Explicitly: In your methods section, do not just state what you did. Note which aspects were optimized, which were based on habit, and which can be flexible. This information is crucial for others attempting to replicate your work.
- Systematically Test Key Parameters: Investigate the robustness of your outcomes to specific variations. For example, test how your results are affected by using different concentrations of high/low nitrate, slight changes in light intensity, or different durations for the recovery step after cutting the primary root [1].
- Adopt FAIR Principles: Ensure your research data and protocols are Findable, Accessible, Interoperable, and Reusable to the greatest extent possible.
Quantitative Data for Benchmarking
Table 1: Protocol Variations in Arabidopsis Split-Root Assays for Nitrate Foraging
This table summarizes the diversity of conditions used in published studies, highlighting parameters that may require benchmarking in your own lab. [1]
| Paper | HN Concentration | LN Concentration | Photoperiod / Light Intensity | Days Before Cutting | Recovery Period | Sucrose in Media |
|---|---|---|---|---|---|---|
| Ruffel et al. (2011) | 5 mM KNO₃ | 5 mM KCl | Long day / 50 mmol mâ»Â² sâ»Â¹ | 8-10 days | 8 days | 0.3 mM |
| Remans et al. (2006) | 10 mM KNO₃ | 0.05 mM KNO₃ | Long day / 230 mmol mâ»Â² sâ»Â¹ | 9 days | None | None |
| Poitout et al. (2018) | 1 mM KNO₃ | 1 mM KCl | Short day / 260 mmol mâ»Â² sâ»Â¹ | 10 days | 8 days | 0.3 mM |
| Tabata et al. (2014) | 10 mM KNO₃ | 10 mM KCl | Long day / 40 mmol mâ»Â² sâ»Â¹ | 7 days | 4 days | 0.5% |
Table 2: Common Root System Architecture Traits for Benchmarking
This table lists key descriptors used to quantify root system architecture, which can be used for comparative analysis. [37] [35] [36]
| Trait Category | Trait Name | Description | Unit |
|---|---|---|---|
| Morphological | Total Root Length | Cumulative length of all roots. | mm |
| Specific Root Length (SRL) | Root length per unit dry mass (often indicates acquisitive strategy). | m/g | |
| Root Tissue Density (RTD) | Root dry mass per unit fresh volume (often indicates conservative strategy). | g/cm³ | |
| Mean Root Diameter | Average diameter of root segments. | mm | |
| Architectural / Topological | Topological Index (TI) | Describes the branching pattern (e.g., herringbone vs. dichotomous). | - |
| Number of Root Tips | Total number of root endings. | - | |
| Lateral Root Density | Number of lateral roots per unit length of parent root. | mmâ»Â¹ |
Experimental Protocols & Workflows
Detailed Protocol: Automated Time-Lapse Imaging of Soil-Grown Root Systems (GLO-Bot)
Application: High-resolution, spatio-temporal quantification of Root System Architecture (RSA) development from germination to maturity in a soil-like environment [39].
Key Materials:
- GLO-Bot Robotic System: A Cartesian gantry robotic system with an imaging arm on a linear rail [39].
- Custom Growth Boxes & Rhizotrons: Designed for hanging in the system and shielding root systems from light [39].
- Luminescent Reporter Lines: Arabidopsis plants expressing constitutively expressed luciferase [39].
- Luciferin Solution: Substrate for luciferase, applied automatically prior to imaging [39].
Methodology:
- Plant Preparation: Sow transgenic seeds in custom rhizotrons filled with a soil-like substrate.
- System Setup: Place up to 84 rhizotrons into the GLO-Bot growth area.
- Automated Workflow:
- The robotic arm moves to a rhizotron, lifts it via a metal hook, and scans its barcode.
- The arm transports the rhizotron to a watering station, where a peristaltic pump slowly applies luciferin solution.
- After watering, the arm places the rhizotron into the imaging chamber (GLO1).
- The system captures both luminescence and standard images from perpendicular cameras.
- The arm returns the rhizotron to its growth box and proceeds to the next one.
- Image Analysis: Use a dedicated pipeline to process time-lapse images. This includes aligning sequential images to boost signal, reducing background noise, and extracting dynamic RSA traits [39].
Workflow Diagram: GLO-Bot Imaging and Analysis Pipeline
Automated RSA Imaging Workflow
Workflow Diagram: Inverse Problem Solving for Root Model Parameterization
Parameter Inference with ABC
The Scientist's Toolkit: Research Reagent Solutions
Table 3: Essential Tools and Reagents for Root Architecture Research
| Item | Function / Application | Key Considerations |
|---|---|---|
| Structural Root Models (ArchiSimple, RootBox) | Generate synthetic root architectures for testing hypotheses and validating image analysis software. | Provides "ground-truth" data; allows exploration of a vast diversity of RSA without physical experiments [37]. |
| Approximate Bayesian Computation (ABC) Framework | Solve the "inverse problem" by inferring mechanistic growth parameters from observed root structures. | Allows for model selection and quantifies uncertainty in parameters without needing a likelihood function [38]. |
| GLO-Roots / GLO-Bot System | Image and quantify soil-grown root systems over time using luminescence reporters and robotics. | Enables time-lapse imaging of mature root systems in conditions closer to natural growth than agar plates [39]. |
| Root System Markup Language (RSML) | Standardized file format for storing and sharing root architecture data. | Promotes interoperability and data reuse by providing a common framework for describing root topologies and geometries [37]. |
| Split-Root Assay Setup | Investigate local vs. systemic signaling in plant responses (e.g., to heterogeneous nutrients). | Critical for nutrient foraging research. Requires careful attention to protocol details like recovery time and nitrate concentrations to ensure robustness [1]. |
Frequently Asked Questions (FAQs)
Q1: What are the core concepts of reproducibility, replicability, and robustness in the context of split-root assays? In experimental biology, reproducibility typically refers to generating quantitatively identical results using the same methods and data. Replicability means that experiments performed under the same conditions produce quantitatively and statistically similar results, which acknowledges biological and experimental noise. Robustness is the capacity to generate similar outcomes even under slight variations in experimental protocol, which is crucial for the relevance of biological phenomena in natural, variable conditions [1].
Q2: Why is robustness particularly important for split-root assay protocols? Robust experimental protocols with outcomes that remain stable under slight variations are more likely to be biologically relevant and reproducible in different laboratory settings. This flexibility is vital for enabling research in labs with varying equipment or funding levels. Furthermore, understanding which protocol variations significantly impact outcomes helps researchers optimize their methods for reliable results [1].
Q3: My split-root assay failed to show the expected systemic signaling phenotypes. What are the first parameters I should check? First, verify your nitrogen concentration differences between compartments (HN vs LN). Second, review the developmental timing of your root cutting and the duration of the recovery period before applying heterogeneous treatments. Inadequate differences in nutrient concentration or plant stress from improper de-rooting can obscure systemic signaling [1] [4].
Q4: What are the advantages of partial de-rooting over total de-rooting when establishing a split-root system? Partial de-rooting (cutting approximately half a centimeter below the shoot-to-root junction) is significantly less stressful for the plant compared to total de-rooting. It results in a shorter recovery time, a final rosette area closer to uncut plants, and a much higher survival rate. This makes it the suggested method for establishing split-root systems in small plants like Arabidopsis thaliana [4].
Q5: How can proteomics be used to validate a successful split-root assay? Proteomic analysis can identify specific protein signatures associated with successful systemic signaling. For instance, in a validated assay, you would expect to see proteomic profiles indicative of distinct metabolic alterations in response to heterogeneous nutrient supply. This can confirm that the local treatment triggered a systemic response captured at the whole-plant level [4].
Troubleshooting Guides
Issue 1: Lack of Preferential Root Foraging Response
Problem: The expected preferential investment in root growth on the high-nitrate (HN) side of the split-root system is not observed.
Solutions:
- Confirm Nitrogen Concentration and Form: Ensure a significant contrast exists between your High Nitrogen (HN) and Low Nitrogen (LN) treatments. The table below shows the variability in published protocols [1].
Optimize the Recovery Period: Allow sufficient time for the plant to recover from the de-rooting stress and develop new roots before applying the heterogeneous nutrient treatment. A recovery period of several days is often necessary [1] [4].
Verify Light and Sucrose Conditions: Inconsistent light intensity, photoperiod, or the presence/absence of sucrose in the growth media can impact plant energy status and root growth responses. Standardize these conditions based on established protocols [1].
Issue 2: Low Plant Survival Rate After De-rooting
Problem: A high percentage of plants die following the surgical procedure to create the split-root system.
Solutions:
- Switch to Partial De-rooting: Immediately adopt the partial de-rooting method instead of total de-rooting. This dramatically increases survival rates [4].
Perform De-rooting at the Right Developmental Stage: The plant's age at cutting critically affects survival. For Arabidopsis, cutting at 11 or 15 days after sowing (DAS) can lead to extended recovery times and decreased final rosette area. Test the optimal timing for your specific plant species and growth conditions [4].
Ensure Aseptic Technique (for in vitro work): Contamination during the surgical step can cause plant death. Maintain sterile conditions throughout the procedure.
Issue 3: High Variability in Proteomic Data
Problem: Proteomic results from replicate samples are inconsistent, making it difficult to identify robust biomarkers.
Solutions:
- Standardize Sample Collection: Collect leaf or root samples at the same time of day and from the same developmental stage to minimize biological variation.
- Implement Rigorous Data Preprocessing: For mass spectrometry-based proteomics, apply optimal missing value imputation and normalization methods. The performance of downstream analyses, including deconvolution, heavily depends on proper preprocessing [40].
- Use a Defined Reference Proteome: When interpreting data, leverage existing, well-characterized immune cell or tissue-specific reference proteomes to improve the accuracy of protein identification and quantification [40].
Experimental Protocols & Data
Standardized Split-Root Protocol for Systemic Signaling
This protocol is optimized for Arabidopsis thaliana based on reviewed methodologies [1] [4].
1. Plant Growth and De-rooting:
- Germinate seeds on standard growth media.
- At 7-10 days after sowing (DAS), perform partial de-rooting. Using a sterile scalpel, make a single cut approximately 0.5 cm below the shoot-to-root junction.
- Transfer the plant to fresh media and allow it to recover for 4-8 days. During this period, new lateral roots will emerge from the remaining root stub.
2. Split-Root Establishment:
- Once two new lateral roots of sufficient length (typically >1 cm) have developed, carefully guide each root into a separate compartment of your split-root device (e.g., a divided petri dish or two separate pots).
- Allow the roots to acclimate for 2-3 days with both compartments containing the same standard growth medium.
3. Heterogeneous Treatment Application:
- Apply the experimental treatment (e.g., High Nitrate (HN) solution) to one compartment and the control (e.g., Low Nitrate (LN) solution) to the other.
- Maintain the treatment for 5-7 days, after which physiological and molecular analyses can be performed.
4. Systemic Signal Validation - Proteomic Analysis:
- Harvest leaf tissue from the treated plants and immediately flash-freeze in liquid nitrogen.
- Perform protein extraction using a standard method like SDS-based lysis.
- Prepare samples for mass spectrometry using a filter-aided method (e.g., S-Trap) with LysC/trypsin digestion [40].
- Analyze peptides using Liquid Chromatography-Mass Spectrometry (LC-MS/MS) in Data-Independent Acquisition (DIA) mode for robust quantification [40].
- Process raw data with specialized software (e.g., DIA-NN) and search against the appropriate protein sequence database.
The diagram below outlines the core logic of a split-root assay for validating systemic signals.
Key Protocol Variations in Split-Root Nitrogen Foraging Studies
Table 1: Summary of protocol variations in Arabidopsis split-root assays from key literature. (Adapted from [1])
| Paper | HN Concentration | LN Concentration | Days Before Cutting | Recovery Period | Heterogeneous Treatment Duration | Sucrose in Media |
|---|---|---|---|---|---|---|
| Ruffel et al. (2011) | 5 mM KNO₃ | 5 mM KCl | 8-10 days | 8 days | 5 days | 0.3 mM |
| Remans et al. (2006) | 10 mM KNO₃ | 0.05 mM KNO₃ | 9 days | None | 5 days | None |
| Poitout et al. (2018) | 1 mM KNO₃ | 1 mM KCl | 10 days | 8 days | 5 days | 0.3 mM |
| Tabata et al. (2014) | 10 mM KNO₃ | 10 mM KCl | 7 days | 4 days | 5 days | 0.5% |
The Scientist's Toolkit: Research Reagent Solutions
Table 2: Essential materials and reagents for split-root assays and subsequent proteomic analysis.
| Item | Function/Application | Example/Note |
|---|---|---|
| Agar Plates (in vitro) | Solid support for root growth in controlled conditions. | Allows for precise surgical procedures and visualization of root development. |
| Divided Containers | Physically separate root environments for differential treatments. | Can be custom-made divided petri dishes or pots. |
| KNO₃ / KCl | Key components for creating High Nitrate (HN) and Low Nitrate (LN) treatments. | KCl is often used in the LN compartment to maintain potassium levels and osmotic balance [1]. |
| Sucrose | Carbon source in growth media for in vitro studies. | Concentration varies (0-1%); its presence/absence can significantly affect plant metabolism and results [1]. |
| SDS (Sodium Dodecyl Sulfate) | Powerful detergent for efficient protein extraction from plant tissues [40]. | Used in lysis buffers to denature proteins and solubilize membrane proteins. |
| LysC/Trypsin | Proteolytic enzymes for digesting proteins into peptides for mass spectrometry analysis [40]. | Typically used in a sequential or combined digestion protocol overnight. |
| S-Trap Micro Spin Columns | Efficient device for protein digestion and cleanup, removing contaminants that interfere with MS [40]. | Superior recovery for a wide range of protein sizes and types. |
| LC-MS/MS System | Core platform for identifying and quantifying thousands of proteins in a complex sample [41] [40]. | Systems like Q Exactive HF-X are commonly used with DIA methods for high reproducibility. |
Data Interpretation & Analysis
Workflow for Integrated Physiological and Proteomic Data Analysis
The following diagram outlines the recommended workflow for analyzing data from a split-root assay, from raw data processing to biological insight.
Troubleshooting Common Experimental Scenarios
Table 3: Troubleshooting guide for specific experimental problems and their solutions.
| Problem Scenario | Potential Causes | Recommended Actions |
|---|---|---|
| No difference in root growth between HN and LN sides. | 1. Inadequate nitrate concentration contrast.2. Insufficient treatment duration.3. High background nitrate in "low" compartment. | 1. Increase HN:LN concentration ratio.2. Extend treatment duration by 2-3 days.3. Verify salt purity and media composition. |
| Plants are stunted after recovery period. | 1. Excessive stress from de-rooting procedure.2. Microbial contamination.3. Sub-optimal growth conditions (light, temperature). | 1. Adopt partial de-rooting method [4].2. Improve sterile technique.3. Review and standardize environmental controls. |
| High technical variation in proteomic results. | 1. Inconsistent sample preparation.2. Suboptimal LC-MS/MS performance.3. Improper data normalization. | 1. Use standardized protein extraction kits (e.g., S-Trap) [40].2. Include quality control samples and technical replicates.3. Apply robust normalization algorithms in DIA-NN. |
| Cannot identify known systemic signaling proteins. | 1. Low abundance proteins.2. Incomplete protein database.3. Ion suppression in MS. | 1. Use deeper protein fractionation or enrichment.2. Search against an exhaustive, organism-specific database.3. Optimize chromatography for peptide separation. |
In scientific research, the terms reproducibility, replicability, and robustness have specific, critical meanings. Reproducibility typically refers to the ability to generate quantitatively identical results when using the same original methods, data, and computational codes. Replicability refers to the capacity for experiments performed under the same conditions to produce quantitatively and statistically similar results, a more achievable goal in experimental biology due to inherent biological noise. Robustness, for the purpose of this case study, is defined as the capacity to generate similar experimental outcomes even when subjected to slight variations in protocol conditions, such as changes in nutrient concentrations, light levels, or equipment used [1].
This technical support center document uses the split-root assay for studying preferential nitrate foraging in Arabidopsis thaliana as a case study to explore these concepts. Preferential foraging describes the phenomenon where a plant's root growth is enhanced in high-nitrate patches and repressed in low-nitrate locations, a response that goes beyond simple local nitrate sensing and involves complex systemic signaling [42] [43]. The complexity of multi-step split-root experiments allows for extensive variation in protocols, making them an ideal model to investigate which protocol details are critical for robust outcomes and which variations are buffered against [1]. Ensuring robust protocols enhances the potential for research to be successfully performed in different labs, even those with less funding or different equipment [1].
Core Signaling Pathways in Preferential Nitrate Foraging
The preferential foraging response is governed by an integrated network of local and systemic signaling pathways. Understanding these mechanisms is fundamental to troubleshooting the assay.
The diagram below illustrates the integration of these key signals.
Key Systemic and Local Signals
The robust phenotypic outcome of preferential foraging arises from the interplay of several key signals [42] [43]:
- Systemic Demand Signaling: Under low external nitrate conditions, roots locally produce CEP peptides. These are translocated to the shoot via the xylem, where they bind to CEP receptors (CEPR). This binding triggers the production of CEP DOWNSTREAM (CEPD) glutaredoxins, which travel back to the root via the phloem. This systemic signal upregulates the high-affinity nitrate transporter NRT2.1 specifically in roots encountering high nitrate, stimulating growth [42] [43].
- Systemic Supply Signaling: Cytokinin (CK) is produced in roots in a nitrate-dependent manner and transported to the shoot. This long-distance CK signaling is thought to function as a supply signal that modulates the strength of the demand signaling, preventing excessive preferential foraging when overall nitrate supply is insufficient [42] [43].
- Local Nitrate Sensing and Response: The dual-affinity nitrate transporter NRT1.1 acts as a key local sensor. At low nitrate levels, it functions as an auxin importer, repressing lateral root growth. At high nitrate levels, this repression is lifted, and NRT1.1-mediated nitrate transport enhances auxin signaling through pathways involving AFB3 and ANR1, thereby promoting local lateral root growth [42] [43].
Mutations in any of the key components—NRT1.1, NRT2.1, CK biosynthesis, or CEP signaling—severely reduce or abolish the preferential foraging response, indicating a synergistic network rather than simply additive actions [42] [43].
Comparative Analysis of Published Split-Root Protocols
A survey of the literature reveals extensive variation in the protocols used for split-root assays investigating nitrate foraging. The table below summarizes key parameters from several seminal studies, all of which successfully observed the core preferential foraging phenotype, demonstrating the robustness of this biological phenomenon to specific protocol parameters [1].
Table 1: Summary of Protocol Variations in Arabidopsis Split-Root Nitrate Foraging Assays
| Paper | HN Concentration | LN Concentration | Photoperiod & Light Intensity | Days Before Cutting | Recovery Period | Heterogeneous Treatment | Sucrose in Media |
|---|---|---|---|---|---|---|---|
| Ruffel et al. (2011) | 5 mM KNO₃ | 5 mM KCl | Long day - 50 mmol mâ»Â² sâ»Â¹ | 8-10 days | 8 days | 5 days | 0.3 mM |
| Remans et al. (2006) | 10 mM KNO₃ | 0.05 mM KNO₃ + 9.95 mM Kâ‚‚SOâ‚„ | Long day - 230 mmol mâ»Â² sâ»Â¹ | 9 days | None | 5 days | None |
| Poitout et al. (2018) | 1 mM KNO₃ | 1 mM KCl | Short day - 260 mmol mâ»Â² sâ»Â¹ | 10 days | 8 days | 5 days | 0.3 mM |
| Girin et al. (2010) | 10 mM NHâ‚„NO₃ | 0.3 mM KNO₃ | Long day - 125 mmol mâ»Â² sâ»Â¹ | 13 days | None | 7 days | 1% |
| Tabata et al. (2014) | 10 mM KNO₃ | 10 mM KCl | Long day - 40 mmol mâ»Â² sâ»Â¹ | 7 days | 4 days | 5 days | 0.5% |
| Mounier et al. (2014) | 10 mM KNO₃ | 0.05 mM KNO₃ + 9.95 mM Kâ‚‚SOâ‚„ | Long day - 230 mmol mâ»Â² sâ»Â¹ | 6 days | 3 days | 6 days | Not Specified |
Workflow for a Standardized Split-Root Assay
The following diagram outlines a generalized experimental workflow for establishing a split-root system, integrating common steps from the literature and highlighting critical steps that influence robustness.
Troubleshooting Guide: Common Issues and Solutions
Problem: Low survival rate of plants after the root-cutting step.
- Potential Cause: The total de-rooting method imposes high stress on the plant, especially if performed at a suboptimal developmental stage [4].
- Solution: Implement a partial de-rooting method, where the cut is made approximately 0.5 cm below the shoot-to-root junction, leaving part of the main root attached. This method leads to a significantly shorter recovery time, a higher survival rate, and a final rosette area closer to that of uncut plants compared to total de-rooting [4].
Problem: High variability in root growth asymmetry between experimental replicates.
- Potential Cause 1: Inconsistent nitrogen sources or osmolarity balances between high nitrate (HN) and low nitrate (LN) treatments. For example, some protocols use KCl in the LN treatment to maintain potassium levels, while others use Kâ‚‚SOâ‚„ [1].
- Solution 1: Closely follow the specific salt compositions from a established protocol (see Table 1) and ensure osmolarity is balanced between HN and LN treatments to avoid water potential effects.
- Potential Cause 2: Inadequate recovery period after cutting before applying the heterogeneous nitrate treatment. The plant needs time to establish new lateral roots.
- Solution 2: Ensure a consistent and sufficient recovery period (typically 3-8 days, see Table 1). Visually confirm that new lateral roots have grown to a sufficient length (e.g., 1-2 cm) before initiating the treatment.
Problem: Weak or absent preferential foraging phenotype.
- Potential Cause 1: The concentration difference between HN and LN patches is insufficient to elicit a strong systemic signal.
- Solution 1: Ensure a large enough difference in nitrate concentrations. Common pairs are 10 mM vs. 0.05 mM or 1 mM vs. 0 mM (with compensating salts) [1].
- Potential Cause 2: The presence of sucrose in the growth media can potentially mask or alter systemic nutrient signaling.
- Solution 2: Consider using a protocol without sucrose (e.g., Remans et al. 2006) to eliminate this variable, but be aware this may slow overall growth [1].
- Potential Cause 3: Genetic background of Arabidopsis plants or mutations in key signaling components (NRT1.1, NRT2.1, CEPR, etc.).
- Solution 3: Use validated wild-type ecotypes (e.g., Col-0) and genotypically confirm your seed stocks.
Frequently Asked Questions (FAQs)
Q1: What is the single most important factor for improving the replicability of my split-root assays? A1: Detailed protocol documentation and adherence. The complexity of these multi-step assays means that seemingly minor unpublished details (e.g., exact agar batch, precise timing of transfers, water quality) can be decisive for success. Meticulously record and follow every step to ensure internal replicability before investigating robustness to variation [1].
Q2: How does partial de-rooting specifically improve my experiment? A2: Research shows that partial de-rooting (PDR) minimizes plant stress compared to total de-rooting (TDR). This results in a shorter recovery time, allowing you to establish the split-root system in younger plants, and a significantly higher survival rate. This leads to more robust and reliable data with less experimental drop-out [4].
Q3: Are the observed growth differences due to a direct effect on root growth or an effect on root development? A3: The preferential foraging response involves both the promotion of lateral root elongation in the high nitrate patch and the suppression of lateral root development in the low nitrate patch. The systemic signals affect both emergence and subsequent growth of lateral roots [42].
Q4: My lab has different growth chambers with different light intensities. Will this affect my results? A4: The robust observation of preferential foraging across protocols using light intensities from 40 to 260 mmol mâ»Â² sâ»Â¹ (see Table 1) suggests the core phenotype is robust to this variation. However, for strict quantitative replicability of growth rates, it is essential to standardize and report this parameter.
Essential Research Reagent Solutions
The following table lists key reagents, genetic tools, and their critical functions in studying nitrate foraging.
Table 2: Key Research Reagents for Nitrate Foraging Studies
| Reagent / Material | Function / Role in Assay |
|---|---|
| KNO₃ & KCl/K₂SO₄ | Forms the basis of the High Nitrate (HN) and osmotically balanced Low Nitrate (LN) treatments. |
| Arabidopsis Wild-type (Col-0) | Standard control ecotype for establishing a baseline foraging phenotype. |
| nrt1.1 / chl1 mutants | Lack a key nitrate sensor/transporter; used to dissect the local and systemic roles of NRT1.1. |
| nrt2.1 mutants | Defective in the high-affinity nitrate transporter upregulated by systemic demand signaling; used to validate NRT2.1's role in growth stimulation. |
| cep or cepr mutants | Disrupted in the root-shoot-root demand signaling pathway; used to demonstrate the role of systemic N demand. |
| Cytokinin Biosynthesis Mutants | Used to investigate the role of systemic supply signaling in modulating the foraging response. |
| Solid Agar Media | Provides a transparent, controlled medium for growing Arabidopsis roots, allowing for phenotyping. |
| Vertical Plates | Standard setup for growing plants to encourage root growth along the surface for easy visualization and imaging. |
Scientific progress in both agriculture and biomedicine relies on reproducible and robust research outcomes. The split-root assay is a powerful technique used to study local and systemic signaling in plants, playing a central role in nutrient foraging research and investigations of abiotic stress responses [1]. However, the complexity of these multi-step experiments allows for extensive variation in protocols, creating significant challenges for achieving replicable and robust results [1]. This technical support center addresses these challenges by providing standardized methodologies, troubleshooting guidance, and strategic frameworks to enhance the translational relevance of your split-root research for real-world agricultural and biomedical applications.
Split-Root System Fundamentals
What is a Split-Root System?
A split-root system (SRS) is formed by a plant whose root system has been physically divided into different compartments that are isolated from each other [4]. The primary advantage of this arrangement is that it allows the differential treatment of separate parts of the root system while they share a common aerial part, thus providing a way to simulate the heterogeneity inherent to field conditions [4].
Key Research Applications
Split-root systems have been successfully employed for studying:
- Systemic versus local regulation mechanisms in plant responses to environmental stimuli [4]
- Plant responses to heterogeneous soil conditions including partial root drying, unequal salt distribution, and varied nutrient distribution [9]
- Water uptake and transport mechanisms, particularly in woody plants [7]
- Interactions with microorganisms including mycorrhizal fungi and pathogens [7]
- Ion transport regulation and nutrient foraging behavior [1]
Establishing Split-Root Systems: Methodological Approaches
Comparison of Split-Root Techniques
Table 1: Split-Root System Establishment Methods
| Method | Description | Best Applications | Advantages | Limitations |
|---|---|---|---|---|
| Partial De-rooting (PDR) | Cutting main root approximately 0.5 cm below shoot-to-root junction, leaving part of main root attached [4] | Young Arabidopsis plants; drought experiments | Shorter recovery time; higher survival rate; less stressful for plants [4] | Requires precision cutting; limited to specific developmental stages |
| Total De-rooting (TDR) | Cutting roots at shoot-to-root junction [4] | Species with strong root regeneration capacity | Complete root system division | Extended recovery time; lower survival rates; more stressful for plants [4] |
| Split-Developed Root (SDR) | Dividing developed root system into two parts of comparable size [7] | Woody plants; established root systems | Simple implementation; suitable for testing heterogeneous soil gradients [7] | Limited applicability for plants with taproots; root damage risk |
| Grafting Methods | Horticultural techniques to graft two seedlings using inverted grafting or approach grafting [7] | Taproot species; physiological studies | Enables study of root-shoot signaling; preserves root integrity [9] | Technically demanding; lower survivability rates; skill-dependent [4] |
| Separation of Newly Formed Roots (SNR) | Pruning taproot to induce lateral roots or rooting shoots [7] | Taproot species; uniform root systems | Produces genetically identical plants; suitable for vegetatively propagated species [7] | Physical damage stress; susceptibility to pathogens; root-shoot imbalance [7] |
Detailed Protocol: Partial De-rooting for Arabidopsis
Materials and Reagents:
- Young Arabidopsis seedlings (5-10 days after sowing)
- Sterile surgical blades or fine scissors
- Growth medium (agar or soil)
- Split-root containers or divided pots
Procedure:
- Plant Preparation: Grow seedlings under controlled conditions until they develop a primary root and early lateral roots.
- Precise Cutting: Using a sterile blade, make a clean cut approximately 0.5 cm below the shoot-to-root junction, preserving a portion of the main root.
- Transfer and Establishment: Immediately transfer the partially de-rooted seedling to your split-root system setup.
- Recovery Phase: Maintain high humidity for 2-3 days to support recovery.
- System Development: Allow 7-10 days for lateral roots to develop sufficiently in both compartments [4].
Critical Considerations:
- Perform the procedure at the optimal developmental stage (typically 7-10 days after sowing for Arabidopsis)
- Maintain sterile conditions to prevent pathogen infection
- Monitor recovery through relative growth rate comparisons with uncut plants [4]
Detailed Protocol: Grafting Method for Cotton
Materials and Reagents:
- Uniform cotton seedlings at the two-true-leaf stage
- Sharp blades (e.g., KW-trio 03541)
- Parafilm grafting strips
- Aerated nutrient solution
- Growth chambers with controlled environment
Procedure:
- Seedling Preparation: Grow uniform seedlings to the two-true-leaf stage (approximately 15 days after planting).
- Incision Technique: Make a '/' shaped incision on the hypocotyl 2 cm below the cotyledons, leaving about 1/3 of hypocotyl tissues intact.
- Rootstock Preparation: Cut the top of the rootstock to form a deep 'ʌ' shape at the same hypocotyl position from another seedling.
- Grafting Union: Insert the 'ʌ' section into the '/' incision and wrap closely with Parafilm.
- Post-grafting Care: Transfer grafted seedlings to aerated nutrient solution, maintain high humidity with plastic bags for one week until new leaves emerge [9].
Validation Metrics:
- Survival rate >95% [9]
- Uniform root system development in both halves
- Successful xylem and phloem connections at graft site [9]
Experimental Design for Robustness
Understanding Reproducibility, Replicability, and Robustness
- Reproducibility: The capacity to generate quantitatively identical results when using the same methods and conditions [1]
- Replicability: Producing quantitatively and statistically similar results when experiments are performed under the same conditions [1]
- Robustness: The capacity to generate similar outcomes despite slight variations in experimental conditions [1]
Mini-Experiment Design for Enhanced Robustness
Implementing systematic heterogeneity through a 'mini-experiment' approach can significantly improve the robustness of your findings:
- Population Splitting: Divide your study population into several mini-experiments conducted at different time points
- Controlled Variation: Allow experimental conditions (personnel, temperature, reagent batches) to vary between mini-experiments while keeping them constant within each mini-experiment
- Blocked Design: Organize each mini-experiment as an independent block in your statistical analysis [44]
This approach enhances external validity by mimicking the inevitable variation that occurs between independent studies and improves the likelihood that your findings will hold across different laboratory environments [44].
Troubleshooting Common Experimental Issues
FAQ: Addressing Split-Root Challenges
Q: What is the optimal developmental stage for establishing split-root systems in young plants?
A: The optimal timing depends on the species and method. For Arabidopsis partial de-rooting, 7-10 days after sowing is ideal. Delaying beyond this window significantly reduces final rosette area and extends recovery time. For cotton grafting, the two-true-leaf stage (approximately 15 days after planting) provides the best results [4] [9].
Q: How can I minimize the stress of root division on plant physiology?
A: Partial de-rooting is significantly less stressful than total de-rooting. Proteomic analyses reveal that partial de-rooting triggers distinct metabolic alterations compared to total de-rooting, resulting in shorter recovery times and final rosette areas much closer to those of uncut plants [4]. Additionally, ensuring minimal root damage during division and maintaining optimal post-procedure humidity reduces stress.
Q: What survival rates should I expect with different split-root techniques?
A: Survival rates vary considerably by method:
- Partial de-rooting (Arabidopsis): High survival rates with proper technique [4]
- Grafting methods (Cotton): >95% with optimized protocols [9]
- Total de-rooting: Significantly reduced survival rates, particularly when performed at suboptimal developmental stages [4]
Q: How can I verify the success of my split-root system before beginning treatments?
A: Assess these parameters:
- Recovery time (time until plants regain growth rates equivalent to uncut controls)
- Root architecture symmetry between compartments
- Absence of visual stress symptoms (wilting, chlorosis)
- For grafting methods, new leaf emergence indicates successful union [4] [9]
Q: What are the key sources of variation that affect replicability in split-root experiments?
A: Major sources of variation include:
- Developmental stage at root division
- Recovery period duration
- Light intensity and photoperiod
- Nutrient media composition (including sucrose concentration)
- Temperature fluctuations [1]
Table 2: Documented Protocol Variations in Split-Root Nitrogen Foraging Studies
| Protocol Parameter | Range of Variations | Impact on Outcomes |
|---|---|---|
| Nitrogen Concentrations | HN: 1-10 mM; LN: 0.05-10 mM [1] | Affects magnitude of preferential foraging response |
| Photoperiod & Light Intensity | Long day (40-230 mmol mâ»Â² sâ»Â¹) to short day (260 mmol mâ»Â² sâ»Â¹) [1] | Influences photosynthetic capacity and root growth |
| Recovery Period | 0-8 days between root division and treatment [1] | Critical for plant recovery and subsequent treatment responses |
| Sucrose in Media | 0-1% concentration [1] | Affects plant carbon status and root growth dynamics |
| Experimental Duration | 5-7 days of heterogeneous treatment [1] | Determines observable phenotypic responses |
Research Reagent Solutions
Table 3: Essential Materials for Split-Root Experiments
| Reagent/Equipment | Specification | Function | Protocol Examples |
|---|---|---|---|
| Growth Media | Various compositions (with/without sucrose) | Root environment; nutrient source | Arabidopsis: 0.3-1% sucrose; Cotton: Aerated nutrient solution [1] [9] |
| Nitrogen Sources | KNO₃, NH₄NO₃, KCl controls | High/Low nitrogen treatments | HN: 1-10 mM KNO₃; LN: 0.05 mM KNO₃ + balance K₂SO₄ [1] |
| Surgical Tools | Sterile blades (e.g., KW-trio) | Precision cutting of roots | Cotton grafting: '/' shaped incision on hypocotyl [9] |
| Grafting Materials | Parafilm strips | Graft union protection | Wrapping graft sites to maintain integrity and prevent desiccation [9] |
| Container Systems | Divided pots, net pots, agar plates | Root compartment separation | Enables differential treatment of root halves [4] |
| Aeration Systems | Aeration instruments | Oxygenation of hydroponic solutions | Critical for grafted seedling survival in hydroponics [9] |
Visualization of Split-Root Experimental Workflows
Split-Root Experimental Workflow with Robustness Enhancement
Advanced Applications and Translational Considerations
Translational Applications in Agriculture
Split-root systems provide unique insights for agricultural applications:
- Water Management: Study partial root-zone drying to develop irrigation strategies that improve water use efficiency without significant yield reduction [7]
- Nutrient Management: Investigate root foraging behavior in heterogeneous nutrient environments to optimize fertilizer placement and reduce environmental impact [1]
- Salinity Tolerance: Explore differential root responses to unequal salt distribution to develop crops with improved salinity tolerance [9]
Methodological Innovations for Enhanced Phenotyping
Recent advances in root system phenotyping technologies offer opportunities for enhancing split-root studies:
- X-ray Computed Tomography: Enables non-destructive, three-dimensional visualization of root system architecture in soil [45]
- High-throughput Visualization: RSAvis3D method allows phenotyping of large populations necessary for genetic analyses [45]
- 4-D Root System Monitoring: Time-series observation of root development in response to heterogeneous conditions [45]
Improving the robustness and replicability of split-root assays requires systematic attention to methodological details and conscious incorporation of heterogeneity in experimental designs. By implementing the standardized protocols, troubleshooting guidelines, and robustness strategies outlined in this technical support resource, researchers can significantly enhance the translational relevance of their findings for both agricultural and biomedical applications. The mini-experiment approach, combined with careful method selection based on plant species and research objectives, provides a pathway to more reliable and generalizable results that bridge the gap between laboratory findings and real-world applications.
Conclusion
Optimizing split-root assays for replicability and robustness is not merely a technical exercise but a fundamental requirement for generating scientifically sound and impactful research. By embracing a holistic approach that integrates precise methodology, a deep understanding of potential stressors, and rigorous validation, researchers can transform this powerful technique into a reliable engine for discovery. The future of split-root research lies in the community-wide adoption of detailed reporting standards and the systematic investigation of how protocol variations influence outcomes. This will unlock the full potential of split-root systems to decipher complex plant signaling pathways, with significant implications for improving crop nutrient use efficiency, understanding plant-environment interactions, and informing the development of therapeutics derived from plant compounds.
References
- 1. Ensuring robustness in scientific research, split-root assays as ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC12451248/]
- 2. Replication Versus Reproduction - by Ben Recht [https://www.argmin.net/p/replication-versus-reproduction]
- 3. Understanding Reproducibility and Replicability - NCBI - NIH [https://www.ncbi.nlm.nih.gov/books/NBK547546/]
- 4. Split-root systems: detailed methodology, alternative ... [https://plantmethods.biomedcentral.com/articles/10.1186/s13007-020-00706-1]
- 5. Ten simple rules for designing and conducting undergraduate ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC10019630/]
- 6. Development of split-root assays for loblolly pine (Pinus taeda ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC9939713/]
- 7. Split-root system as a useful tool to study woody plant biology [https://link.springer.com/article/10.1007/s11104-023-06025-3]
- 8. Unraveling the hydrodynamics of split root water uptake ... [https://www.frontiersin.org/journals/plant-science/articles/10.3389/fpls.2015.00370/full]
- 9. Establishment of New Split-root System by Grafting [https://en.bio-protocol.org/en/bpdetail?id=2136&type=0]
- 10. Establishment of a rapid split-root assay in hydroponic ... [https://www.sciencedirect.com/science/article/pii/S2215016125005588]
- 11. Protocol for analyzing root halotropism using split-agar ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC10025266/]
- 12. Establishment of a rapid split-root assay in hydroponic ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC12657376/]
- 13. A Scalable Hydroponic System for Precise Nutrient and ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC12288440/]
- 14. Evaluation of Split Root Nutrient Film Technique (SR-NFT) ... [https://www.mdpi.com/2077-0472/15/13/1350]
- 15. Optimizing Protocols for Arabidopsis Shoot and Root ... [https://www.mdpi.com/2223-7747/10/2/375]
- 16. Regulation of Resource Partitioning Coordinates Nitrogen ... [https://www.sciencedirect.com/science/article/pii/S1674205219301273]
- 17. Development of split-root assays for loblolly pine (Pinus ... [https://www.sciencedirect.com/science/article/pii/S2215016123000493]
- 18. Studies of rhizobial competitiveness for nodulation in ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC6605011/]
- 19. Unveiling the microbiome of hydroponically cultivated lettuce [https://pmc.ncbi.nlm.nih.gov/articles/PMC10872686/]
- 20. Nanoplastics indirectly compromise lettuce growth in ... [https://www.sciencedirect.com/science/article/pii/S030438942403036X]
- 21. Technical FAQ - Demo BeCrop® Technology [https://info.biomemakers.com/support/technical-faq]
- 22. Hydroponics [https://extension.okstate.edu/fact-sheets/hydroponics.html]
- 23. Hydroponics: A Versatile System to Study Nutrient ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC5091364/]
- 24. How to Improve Hydroponic Gardening [https://drygair.com/blog/hydroponic-greenhouse/]
- 25. Recent Advances in Legume-Microbe Interactions [https://pmc.ncbi.nlm.nih.gov/articles/PMC1914196/]
- 26. Split-root systems: detailed methodology, alternative applications, and implications at leaf proteome level - PMC [https://pmc.ncbi.nlm.nih.gov/articles/PMC7797125/]
- 27. Significance of Root Severance on Performance of Established Trees | Arboriculture & Urban Forestry [https://auf.isa-arbor.com/content/14/12/288]
- 28. Ensuring robustness in scientific research, split-root assays as ... [https://resolve.cambridge.org/core/journals/quantitative-plant-biology/article/ensuring-robustness-in-scientific-research-splitroot-assays-as-an-example-case/047641FE2573A6FE0B4A22A7FF06A925]
- 29. Ensuring robustness in scientific research, split-root assays ... [https://pubmed.ncbi.nlm.nih.gov/40989016/]
- 30. Comparing root exudate collection techniques - PubMed Central [https://pmc.ncbi.nlm.nih.gov/articles/PMC8444088/]
- 31. Alternative Rooting Methods for Medicinal Cannabis ... [https://www.mdpi.com/2223-7747/12/11/2216]
- 32. HPLC Troubleshooting Guide [https://www.thermofisher.com/us/en/home/industrial/chromatography/chromatography-learning-center/high-performance-liquid-chromatography-hplc-support/hplc-troubleshooting.html]
- 33. Good Documentation Practices for a Clinical Trial [https://medinstitute.com/blog/good-documentation-practices-for-a-clinical-trial/]
- 34. Good Documentation Practices (GDP) - Office for Human ... [https://www.rochester.edu/ohsp/resources/study-documentation/gdp/]
- 35. Variations in root architecture traits and their association ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC10847883/]
- 36. Fine Root Traits across Different Root Orders and Their ... [https://www.mdpi.com/2223-7747/13/17/2472]
- 37. Using a Structural Root System Model to Evaluate and ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC5376626/]
- 38. Model selection and parameter estimation for root ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC6685013/]
- 39. Uncovering natural variation in root system architecture and ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC9499532/]
- 40. Considerations and Software for Successful Immune Cell ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC12322953/]
- 41. A plasma proteomics-based candidate biomarker panel ... [https://www.nature.com/articles/s41591-025-03890-6]
- 42. Modeling of Root Nitrate Responses Suggests Preferential ... [https://www.frontiersin.org/journals/plant-science/articles/10.3389/fpls.2020.00708/full]
- 43. Modeling of Root Nitrate Responses Suggests Preferential ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC7268170/]
- 44. Improving reproducibility in animal research by splitting the ... [https://www.nature.com/articles/s41598-020-73503-4]
- 45. High-throughput three-dimensional visualization of root ... [https://plantmethods.biomedcentral.com/articles/10.1186/s13007-020-00612-6]